Cancer precursor project - breast cancer, part 6b
18 November 2024, revised 4 February 2025
In part 6a, we discussed breast cancer mortality, breast cancer risk factors and how breast cancer arises. This essay discusses breast anatomy, histology (microscopic appearance) and breast cancer precursors. We discuss all breast cancers with known precursors, starting with infiltrating duct carcinoma of no special type, lobular carcinoma and pleomorphic lobular carcinoma.
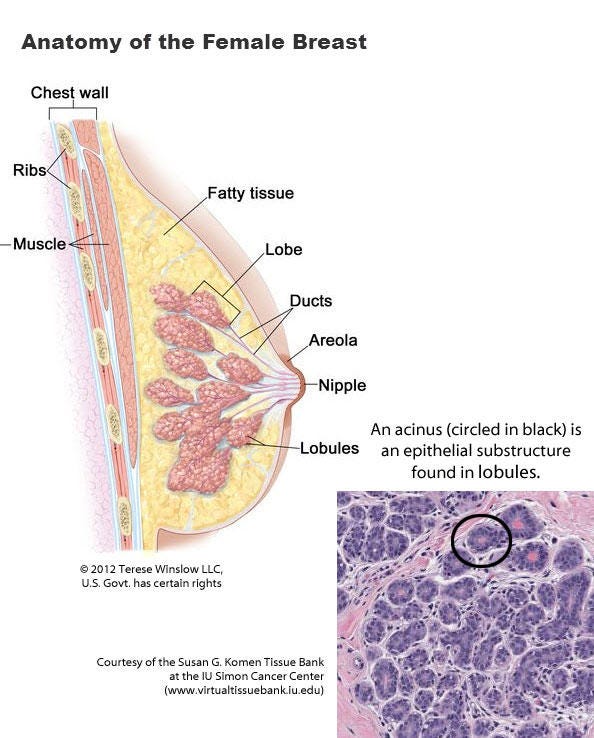
Breast anatomy and histology
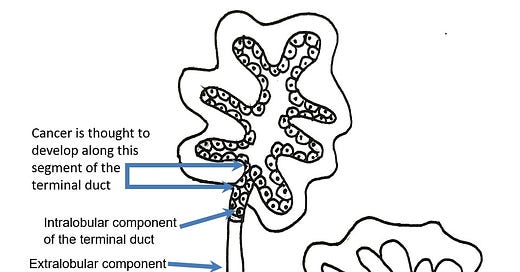
Most cases of breast cancer arise from the progenitor or stem cells of the terminal duct lobular unit, part of the normal anatomy of the female breast:
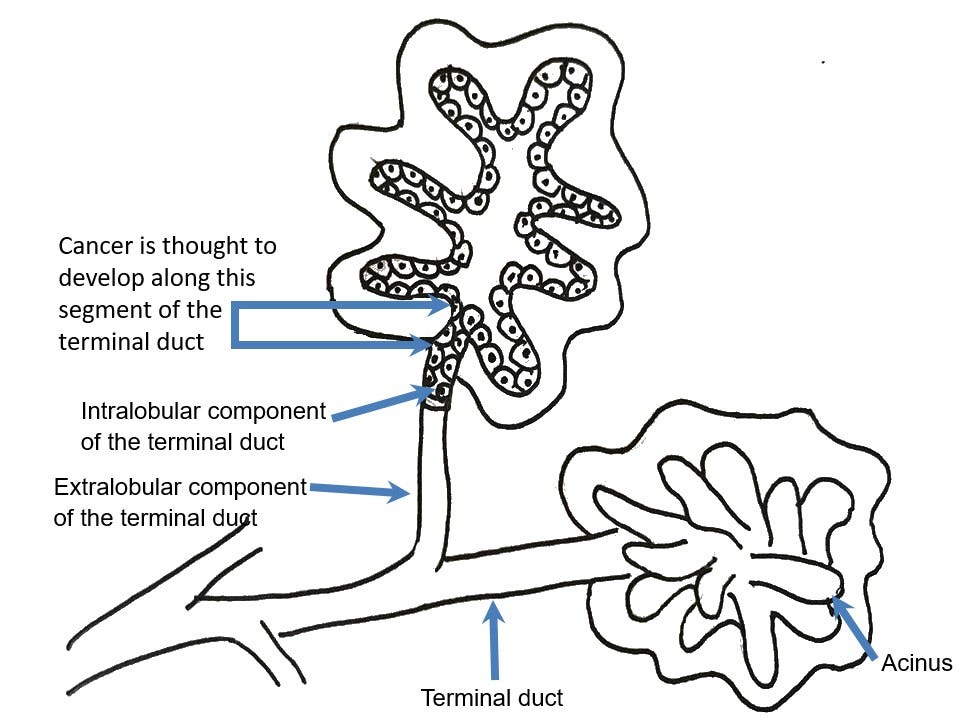
Common breast cancer types are called ductal or lobular for historical reasons - they no longer accurately reflect our understanding of their origin.
Breast cancer precursors

Cancer precursors are the tissue antecedents of cancer. They reflect a morphologic (microscopic shape and structure) rather than a biochemical or genetic association with a specific cancer. Their presence indicates a high likelihood of progression to invasive cancer, usually without a known intermediate step. They typically are identified initially as dysplastic or neoplastic changes adjacent to invasive cancer. The purpose of identifying and studying precursors is to understand how cancer arises, to provide more options to prevent or treat disease (by treating the precursor) and to predict the future of the disease.
As discussed previously, breast cancer risk factors appear to activate four superpromoters of malignant transformation (chronic inflammation, DNA alterations, immune system dysfunction and prolonged hormonal exposure). This may cause breast epithelial cells to transform into the precursors low grade or high grade DCIS. These precursors represent different attractor states that pull in cells with similar network configurations into common microscopic patterns. Attractors tend to maintain cellular network stability against common disruptions, which is why DCIS persists for years even though molecular changes may be ongoing.
In the breast and other epithelia, malignant transformation is slowed by epithelial cell junctions and basement membranes. The epithelial cells may have malignant properties but they are constrained by cell junctions or the presence of basement membranes, which limit invasion until additional molecular changes occur. In contrast, nonepithelial cells can more readily multiply and grow.
Precursors may transform into invasive disease due to the persistent activity of these superpromoters and their own genetic instability. Despite the numerous network changes possible in the transformation, they converge into a limited number of breast cancer types based on the attractor concept.
How breast cancer arises without precursors will be discussed separately.
Our cancer precursor project
Through our cancer precursor project, we have identified 45 distinct types of breast cancer of which 14 have precursors. These essays describe these 14 types of breast cancers with identified precursors beginning with:
Infiltrating duct carcinoma of no special type (very common), precursors are ductal carcinoma in situ (DCIS) and lobular carcinoma in situ (LCIS)-classic.
Lobular carcinoma-classic (very common), precursors are LCIS-classic, florid LCIS, pleomorphic LCIS and atypical lobular hyperplasia (ALH).
Pleomorphic lobular carcinoma (rare variant of lobular carcinoma), precursors are LCIS-classic and pleomorphic LCIS.
Breast carcinoma means that the cancer arises from breast epithelial cells. Breast cancer includes breast malignancies from all types of cells and includes lymphomas, sarcomas or metastases to the breast.
Infiltrating duct carcinoma of no special type
Infiltrating duct carcinoma of no special type is also known as invasive breast carcinoma of no special type and infiltrating duct carcinoma not otherwise specified (NOS). It is the most common type of invasive breast carcinoma (75 - 80% of cases). It is defined as an invasive carcinoma with microscopic or immunohistochemical evidence that it arises in the breast. By definition, it lacks features to qualify as one of the special subtypes of breast cancer listed below in italics from PathologyOutlines.com:
Invasive breast carcinoma of no special type and variants: invasive breast cancer of no special type (NST) medullary NST rare variants
Metaplastic carcinoma: fibromatosis-like low grade adenosquamous metaplastic squamous cell carcinoma
Neuroendocrine carcinoma: neuroendocrine carcinoma (NEC) neuroendocrine tumor
Other invasive carcinoma subtypes, WHO classified: acinic cell carcinoma adenoid cystic apocrine cribriform microinvasive micropapillary mixed NST mucinous mucinous cystadenocarcinoma mucoepidermoid papillary polymorphous secretory tall cell carcinoma with reverse polarity tubular
Other carcinoma subtypes, not WHO classified: BRCA associated carcinoma cystic hypersecretory carcinoma HER2 low breast cancer myoepithelioma and myoepithelial carcinoma tubulolobular carcinoma
Invasive duct carcinoma (IDC) of no special type arises from breast epithelial progenitor or stem cells in the terminal duct lobular unit, as do other types of breast carcinoma. Important prognostic factors are patient age, tumor stage, lymph node status, histologic grade, clinical biomarker profile and tumor gene expression signature. Patients are treated with surgical excision and possibly with radiotherapy, systemic chemotherapy or targeted therapies.
Histologic grade is low (grade 1), intermediate (grade 2) or high (grade 3).
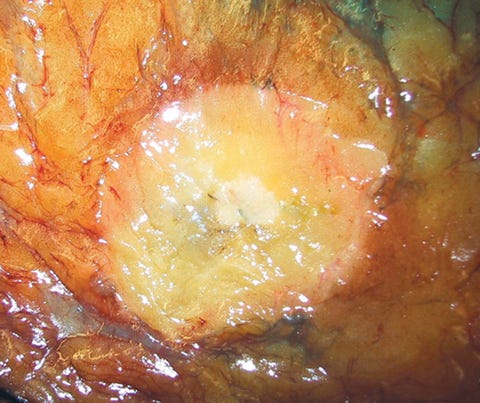
Infiltrating duct carcinoma (IDC) of no special type - microscopic images
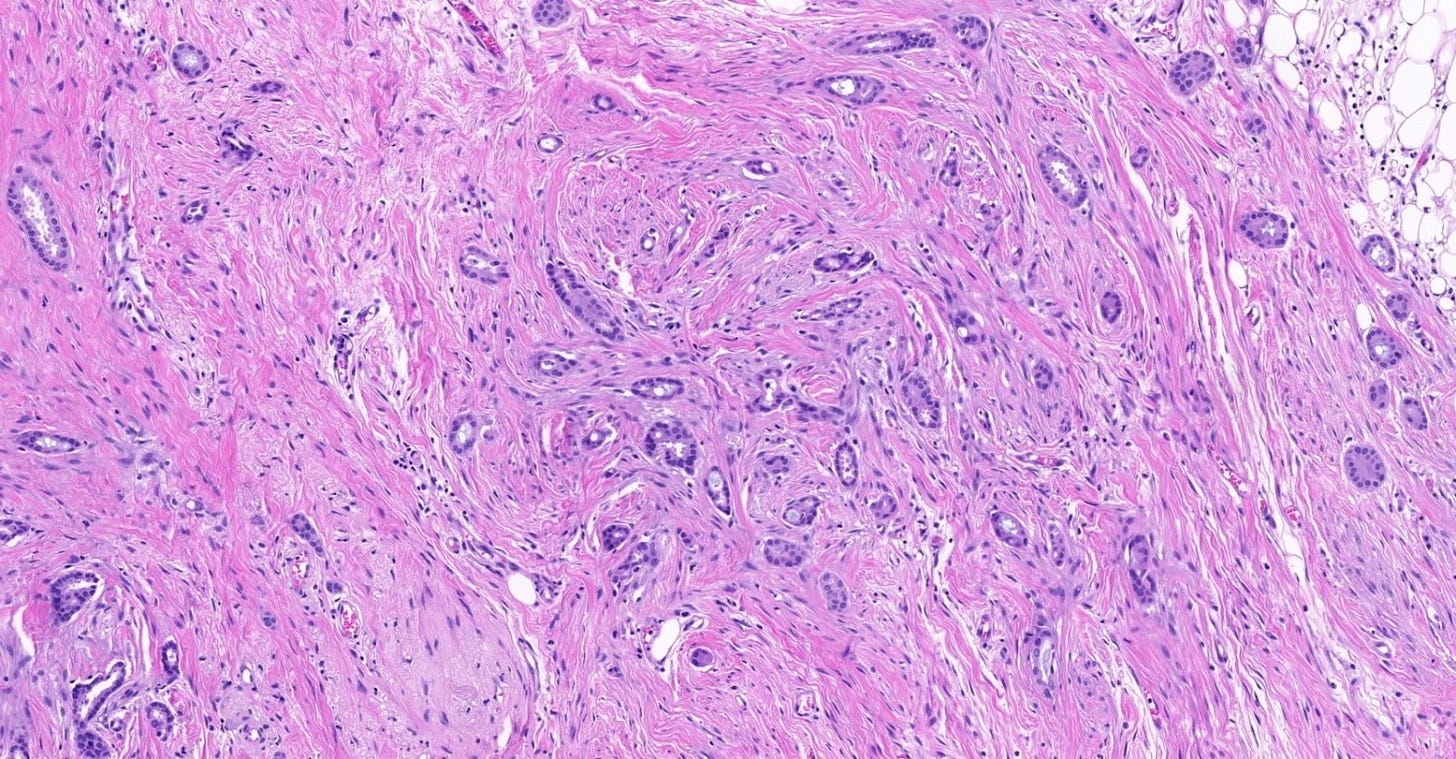
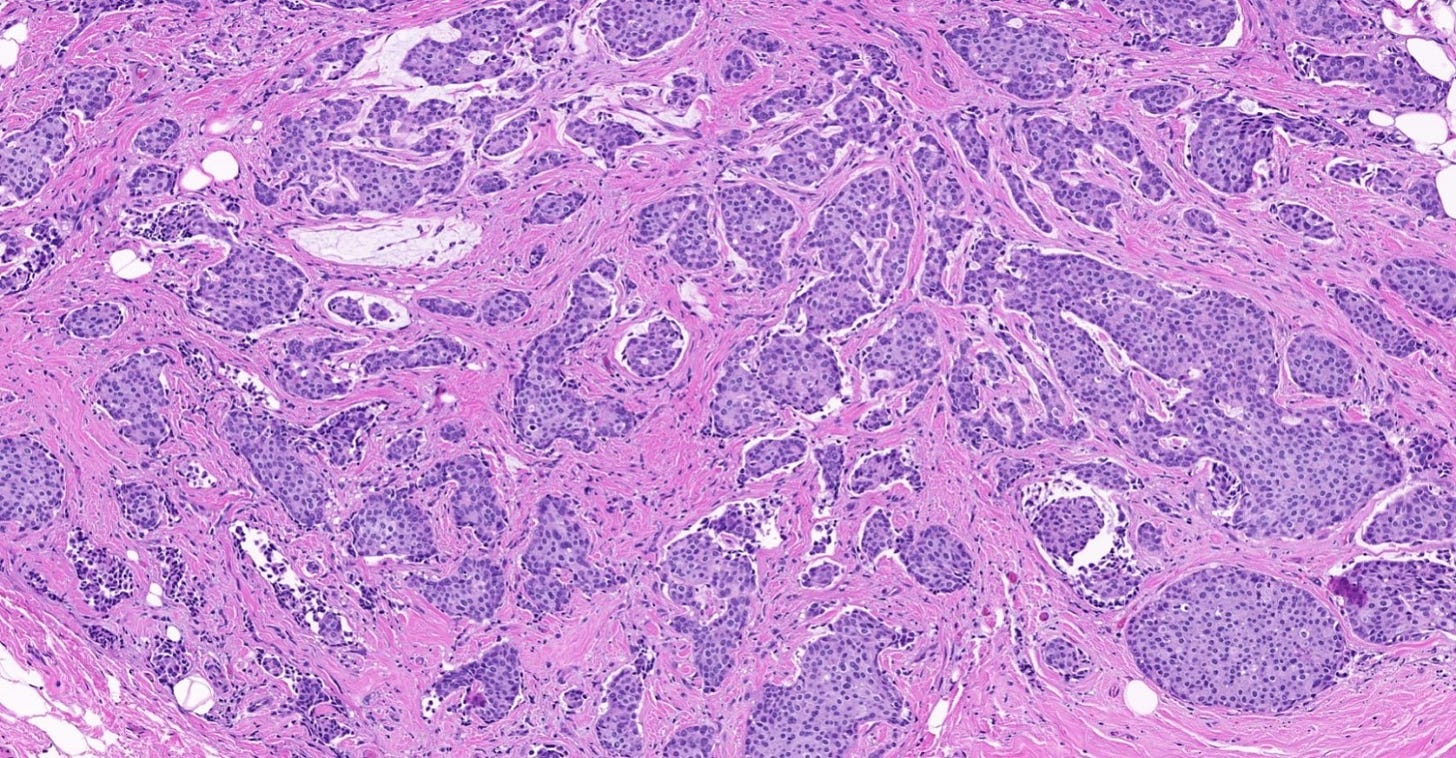
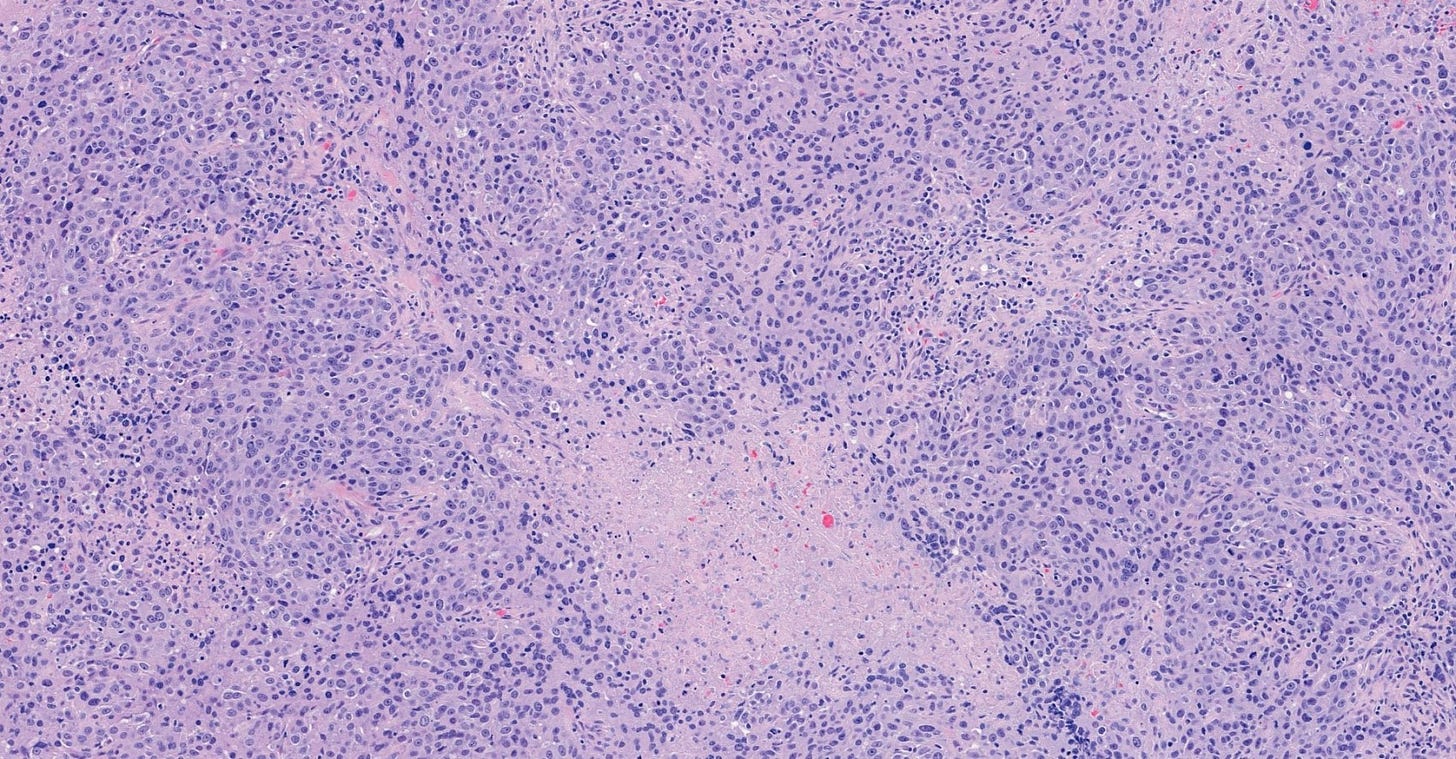
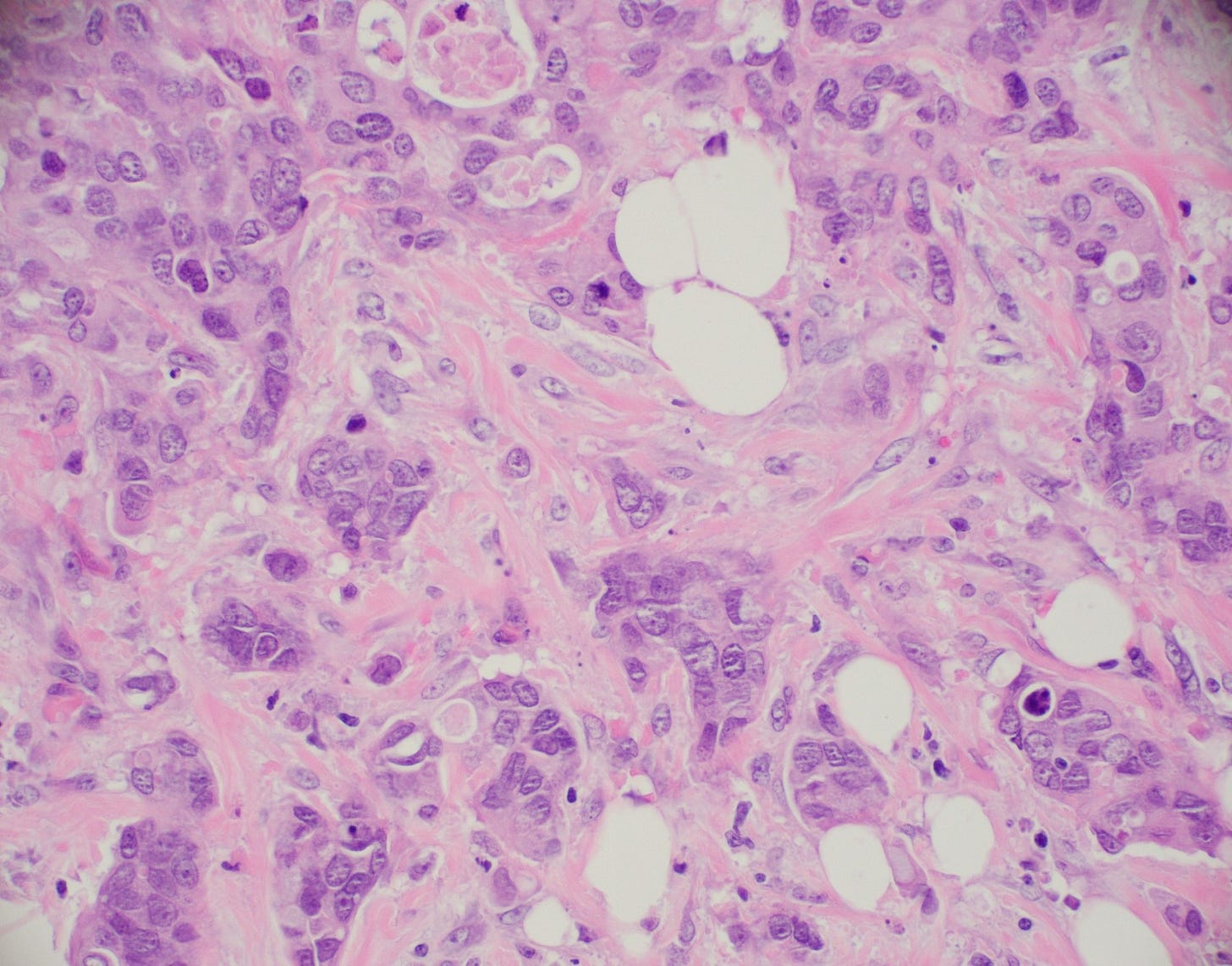
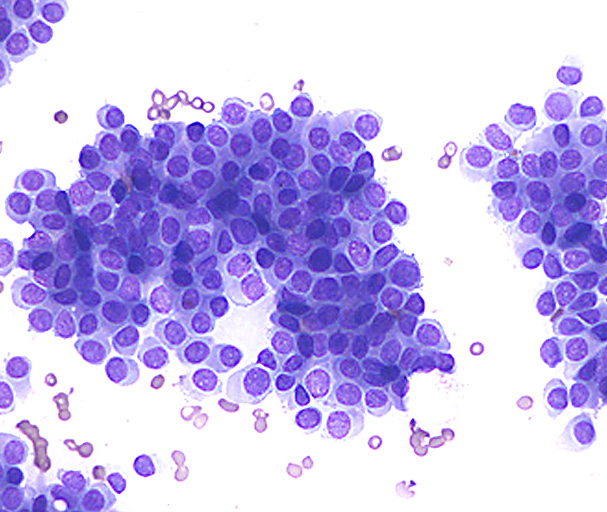
The premalignant precursors of invasive duct carcinoma of no special type are DCIS (ductal carcinoma in situ) and classic LCIS (lobular carcinoma in situ). In addition, we speculate that there may be unidentified precursors based on molecular patterns of change without microscopic changes, although this is unproven.
Precursor of IDC - ductal carcinoma in situ (DCIS)
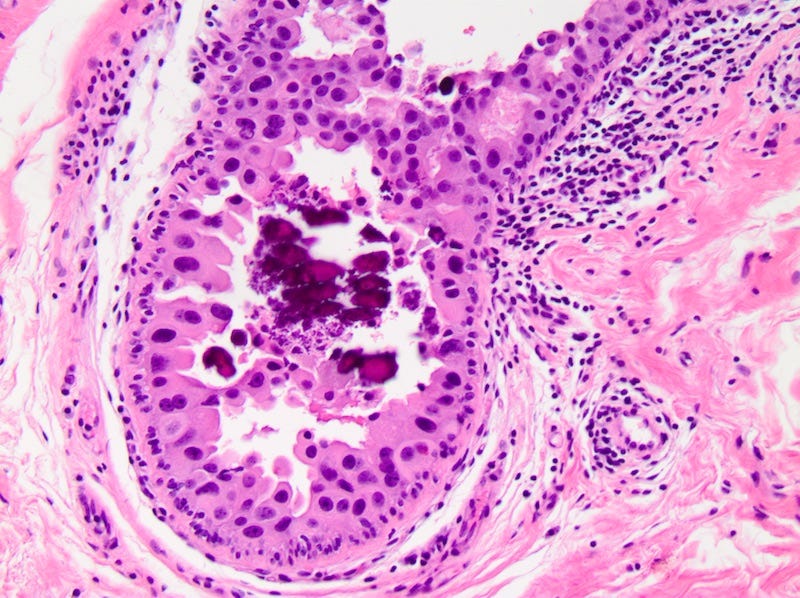
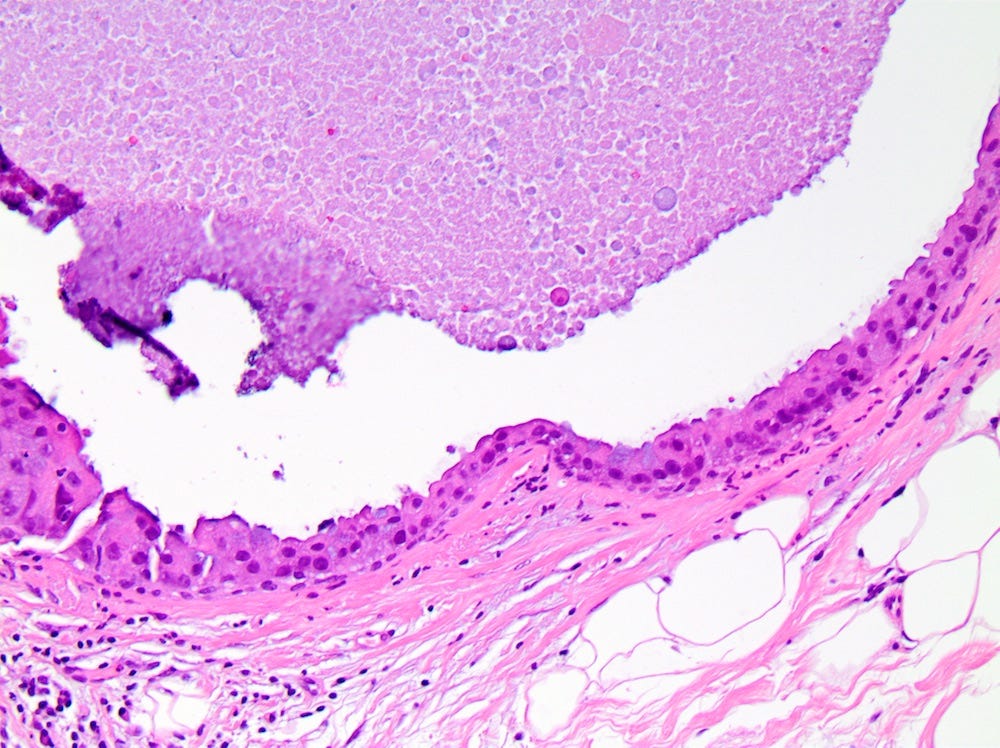
Ductal carcinoma in situ (DCIS) is a neoplastic proliferation of breast ductal epithelial cells within the ductal-lobular system with malignant microscopic features but without evidence of invasion through the epithelial basement membrane or myoepithelial cell layer into the surrounding stroma.
DCIS is a precursor of infiltrating duct carcinoma of no special type. It is also a precursor of tubular carcinoma, cribriform carcinoma, male invasive carcinoma and neuroendocrine carcinoma-small cell type.
DCIS includes a heterogeneous group of lesions in terms of microscopic appearance, molecular alterations, biomarker expression profile and biologic potential for progression to invasive carcinoma. It is divided into low grade (less aggressive) DCIS and high grade DCIS. Normal breast tissue appears to transform to only one of these entities through two distinct molecular pathways (i.e. high grade DCIS does not typically arise from low grade DCIS). Low grade DCIS shows frequent chromosomal losses at 16q. High grade DCIS shows frequent losses at 8p and gains at 17q and has a similar molecular profile as invasive breast cancer.
The microcalcifications that identify DCIS on mammograms are a response to cell death from either a hypoxic environment (the Warburg effect) or cell crowding due to abnormal cells growing unchecked inside the duct. When these calcifications appear on a mammogram, they often have suspicious features that require further investigation.

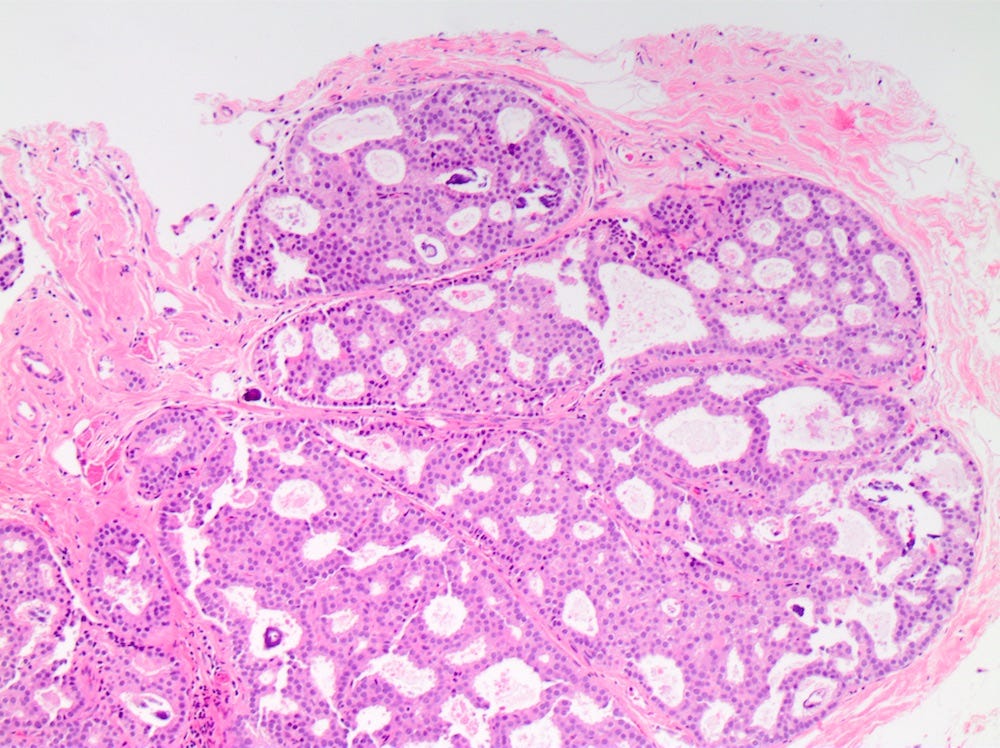
DCIS - microscopic images
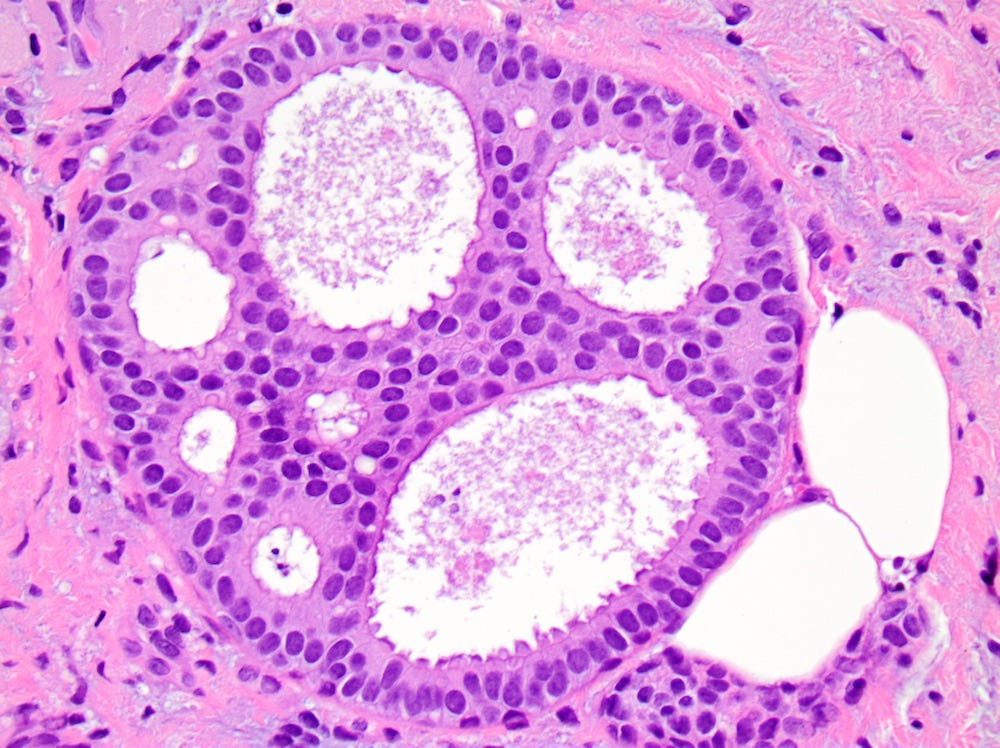
Precursor of IDC - classic lobular carcinoma in situ (LCIS)
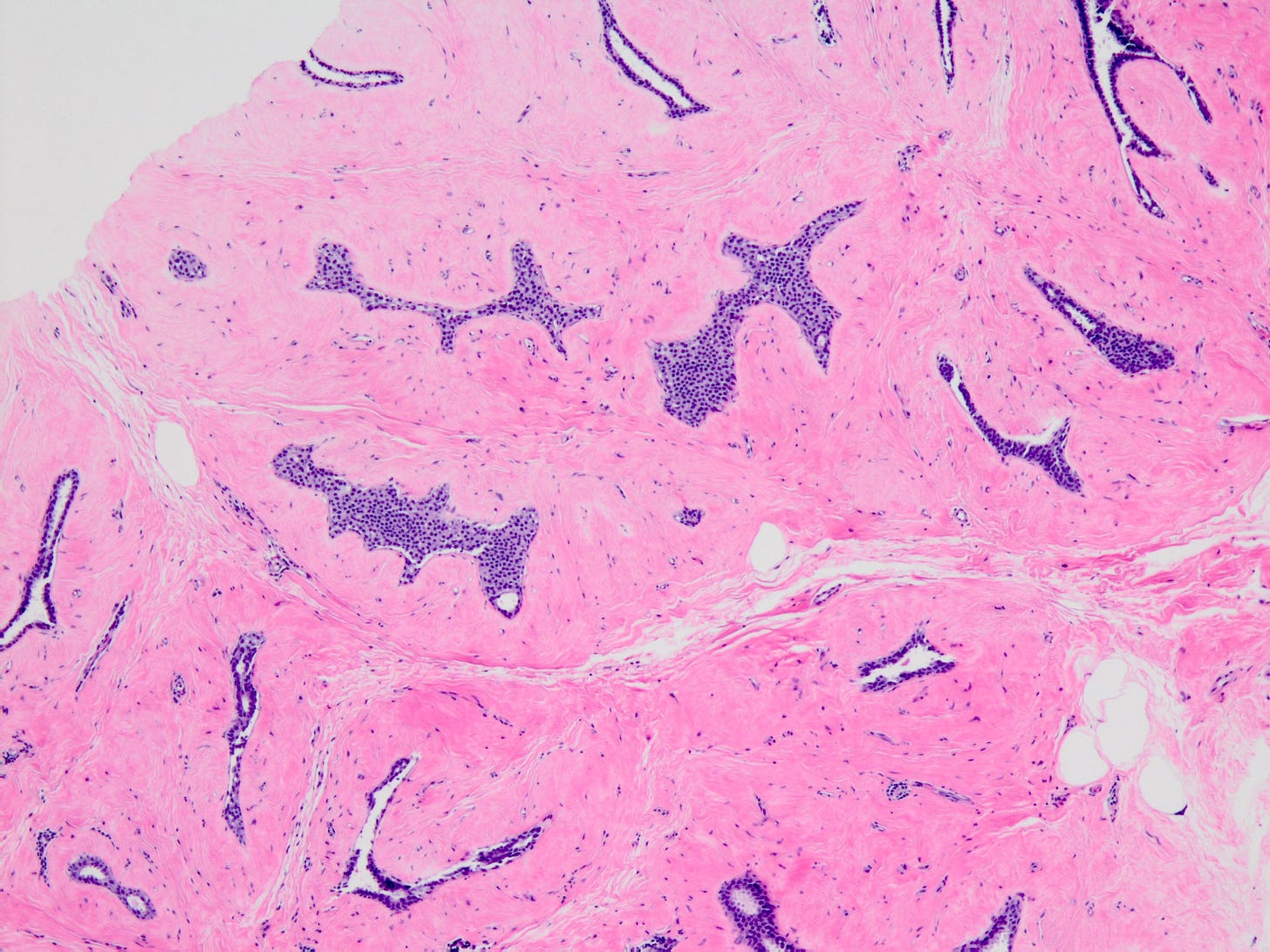
Lobular carcinoma in situ (LCIS) - classic is a precursor of infiltrating duct carcinoma of no special type (IDC), invasive lobular carcinoma-classic and pleomorphic lobular carcinoma. LCIS has two nonclassic variants - LCIS florid and LCIS pleomorphic, that are not precursors of IDC but will be discussed below under lobular carcinoma.
Normal breast cells appear to transform to LCIS based on changes to the E-cadherin gene (mutations, loss of the wild type allele or methylation). E-cadherin mediates intracellular adhesion and cell polarity and plays a key role in maintaining lobular architecture; it can also inhibit the growth and invasion of breast cancer cells. Loss of E-cadherin protein (which occurs in > 95% of cases), or rarely its aberrant expression (due to mutations or loss of the wild type allele), results in loss of cell to cell cohesion, increased cell proliferation and altered organization of the lobules, giving rise to the characteristic microscopic appearance of lobular carcinoma in situ.
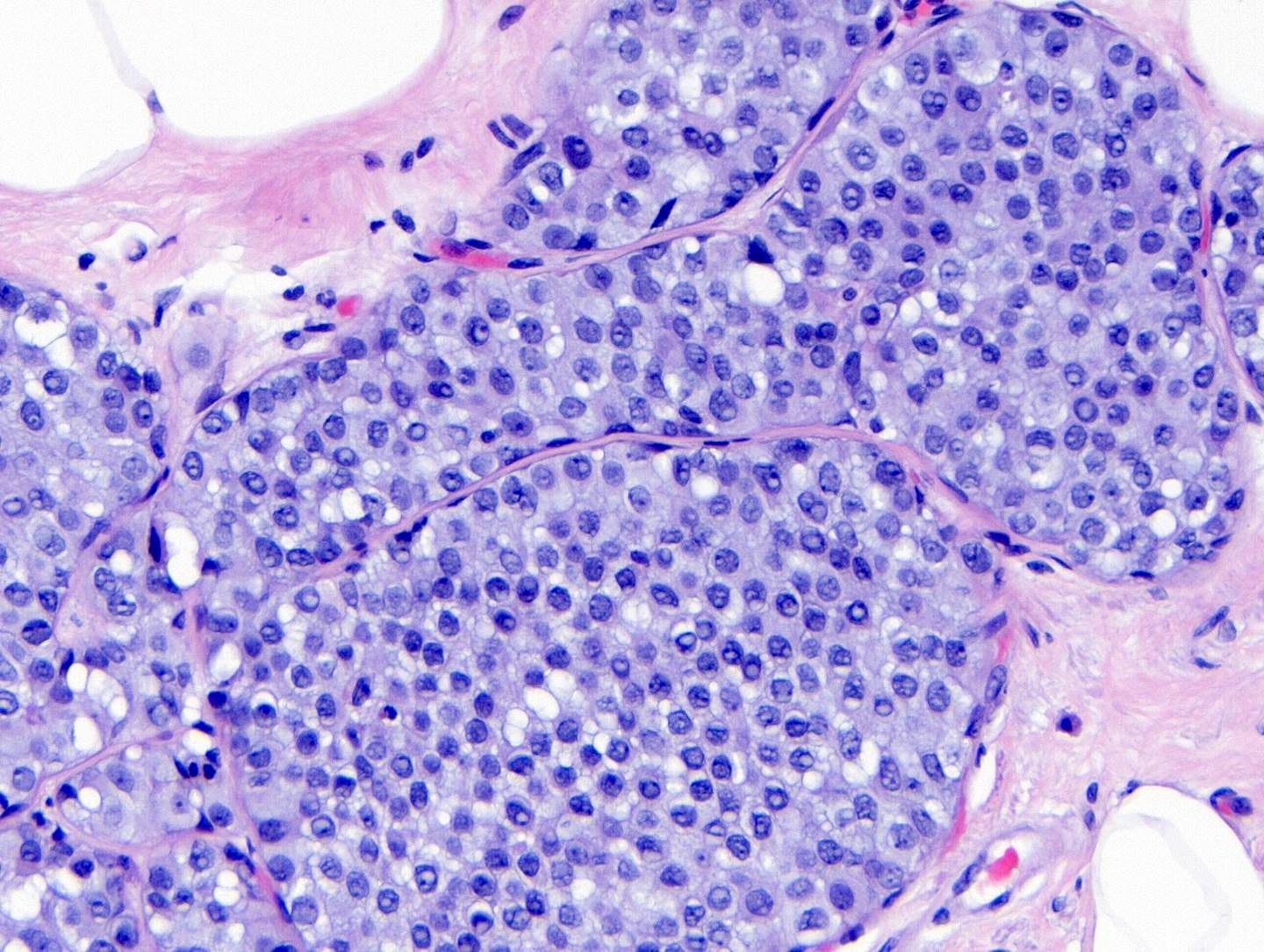
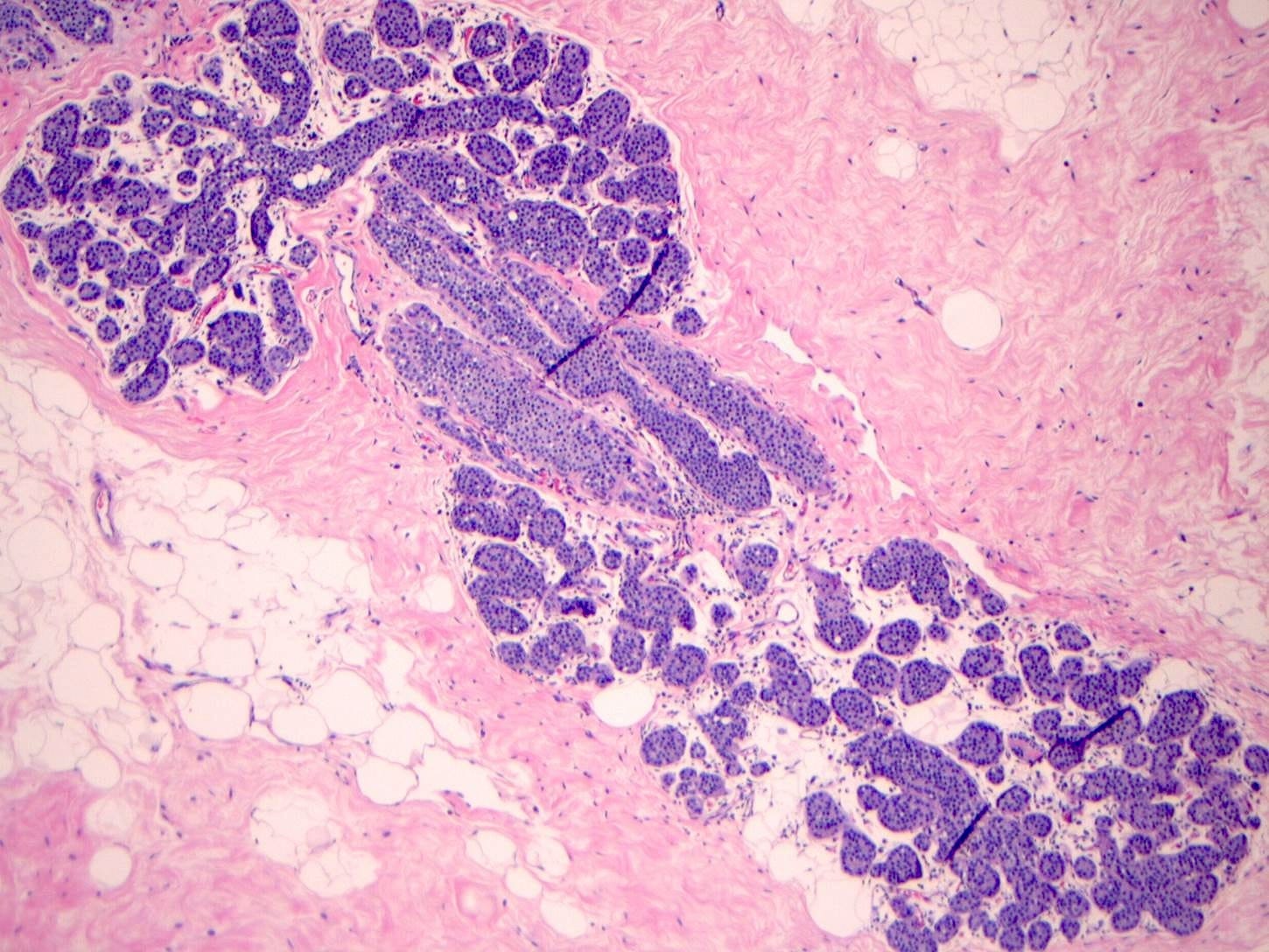
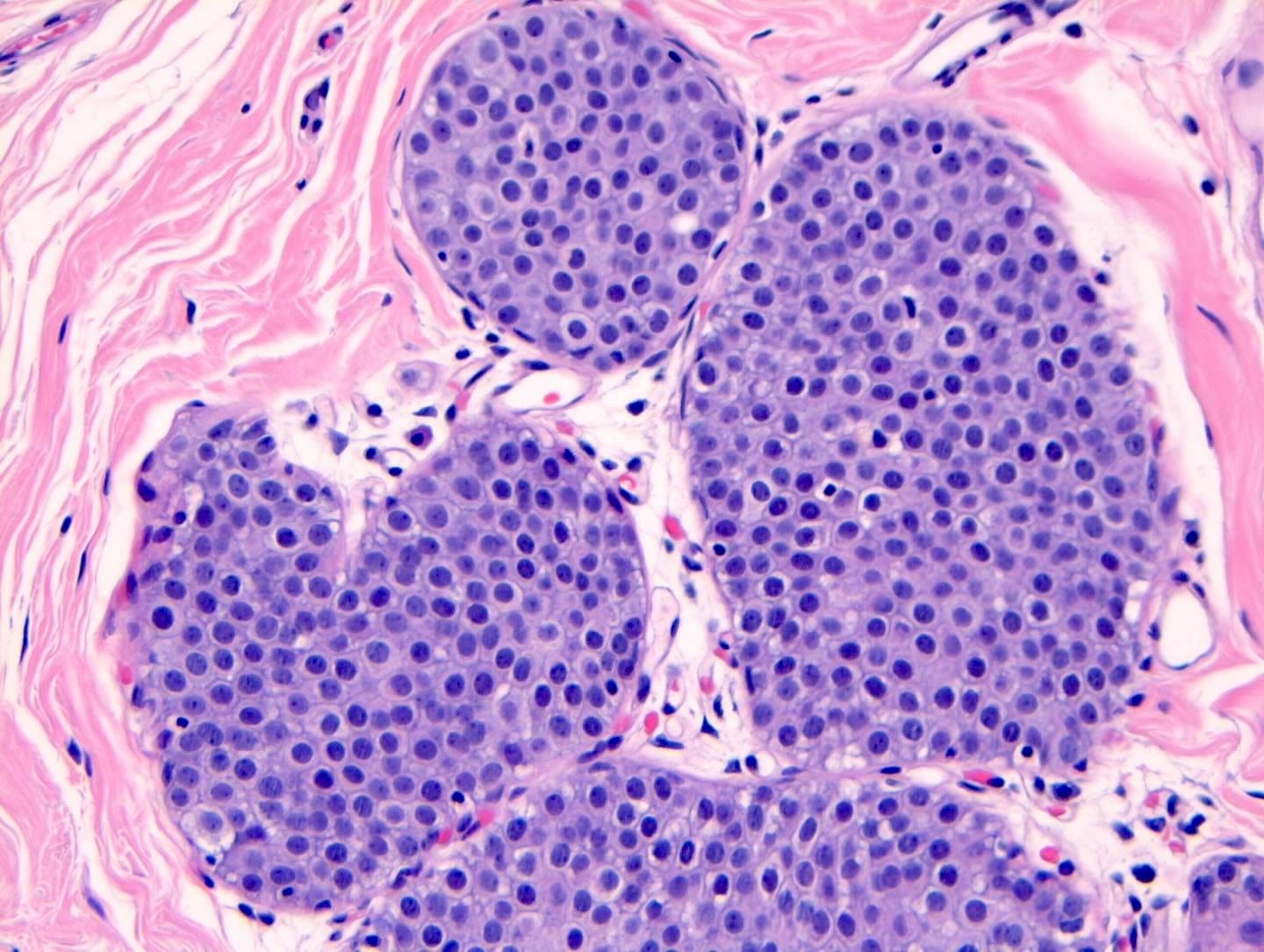
As shown below, LCIS is characterized by a lobulocentric proliferation of cells that expand lobular units. The LCIS cells are uniform, loosely cohesive and evenly spaced (“marbles in a bag”). They are slightly larger than normal breast epithelial cells, with indistinct cell borders and pale cytoplasm. Two types may coexist. Type A LCIS cells have small to slightly enlarged, uniform, round nuclei with inconspicuous nucleoli. Type B cells have larger nuclei, more abundant cytoplasm and more prominent nucleoli.
Classic LCIS is usually an incidental finding in a breast biopsy performed for other indications, including screening detected calcifications or mass producing lesions. The calcifications are frequently produced by columnar cell lesions, a low risk lesion. LCIS lesions are frequently found as multiple foci within the same (70%) or both breasts (20 - 60%).
LCIS is a risk factor for subsequent breast cancer: women with LCIS have a 7 - 10 fold increase in breast cancer risk, an absolute risk of 1 - 2% per year and a lifetime risk of 30 - 40%. The time from LCIS diagnosis to cancer ranges from 15 to 30 years.
Classic LCIS is typically treated conservatively with active surveillance and anti estrogens. Unlike DCIS, it is no longer considered a malignant condition. If diagnosed incidentally, excision is not recommended because the risk of finding invasive carcinoma on excision is very low (< 5%). Excision is recommended only if other high risk proliferative lesions are found or there is discordance between histology and imaging (i.e. the microscopic findings are different from what is expected from the imaging, suggesting the lesion was missed).
Classic LCIS - microscopic images
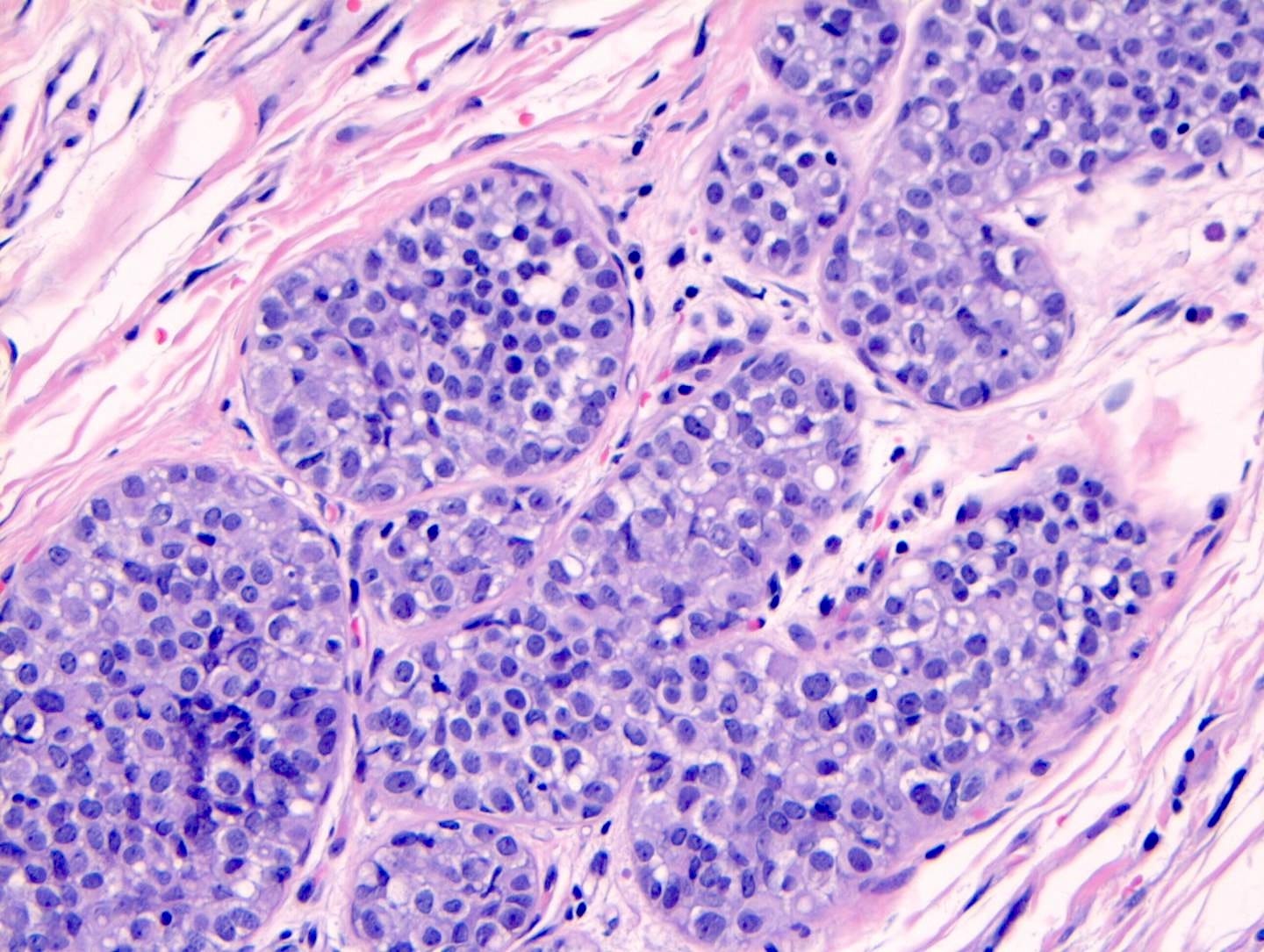

Lobular carcinoma
Lobular carcinoma is a common type of invasive breast carcinoma (10% of cases). It is characterized by the loss of cellular adhesion with dyscohesive tumor cells arranged in single file or as individual single cells. The cells show a loss of 16q (the CDH1 gene located at 16q22.1 encodes E-cadherin, essential in forming the adherens junction responsible for cell adhesion). Loss of E-cadherin protein expression by immunohistochemistry is helpful but not required for diagnosis.
Lobular carcinoma of the breast typically presents with vague findings such as thickening, swelling or a poorly defined breast mass. In general, it is more difficult to detect with mammography (due to infrequent calcifications) and more often presents as a larger tumor with nodal involvement compared to invasive ductal carcinoma of no special type. It is more frequently bilateral and multifocal.
A mastectomy may be preferred because of the risk of local recurrence. These patients generally respond better to hormonal therapy compared to those with invasive ductal carcinoma of no special type. However, their response to radiation therapy is comparable, while their response to chemotherapy is less favorable
Classic lobular carcinoma - microscopic images
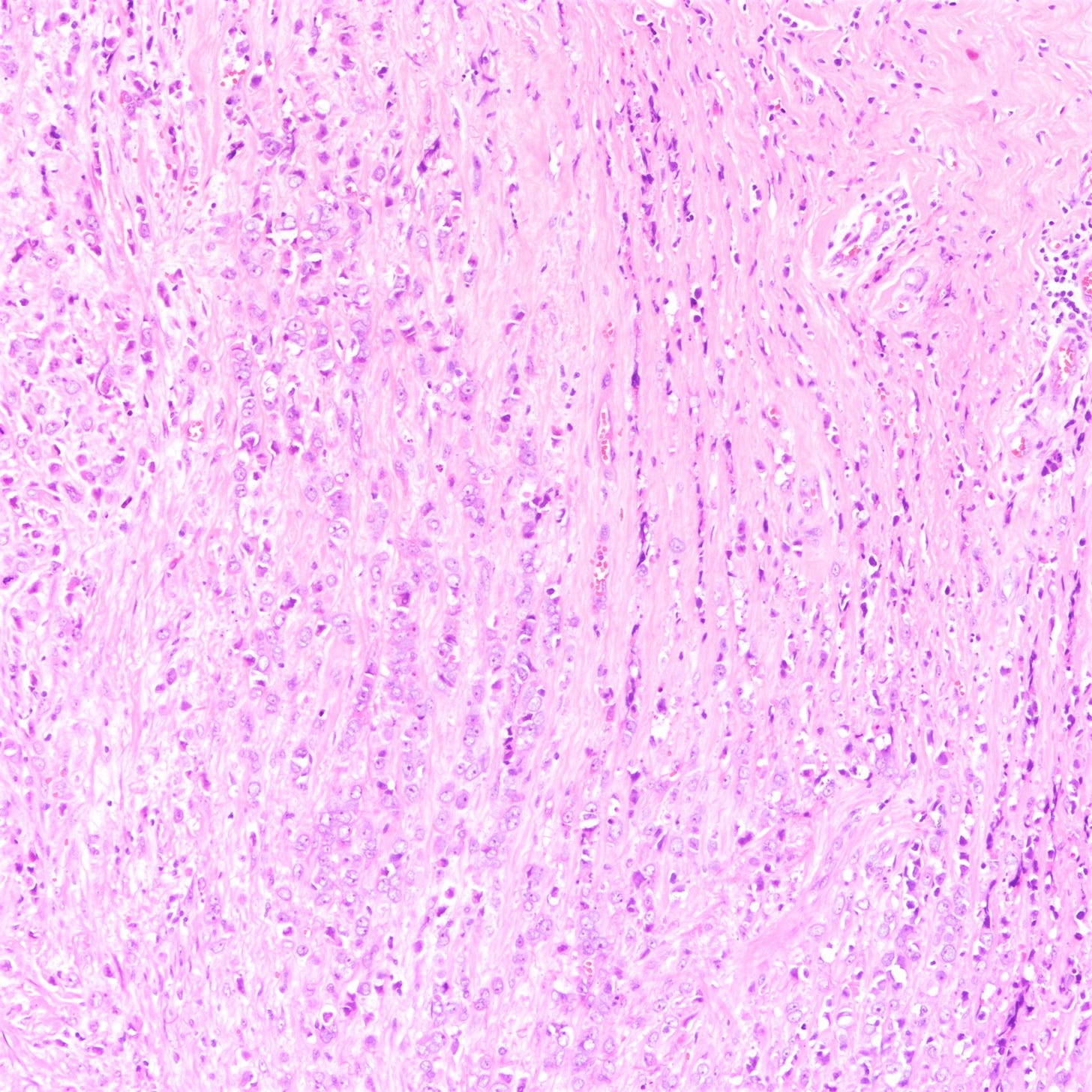
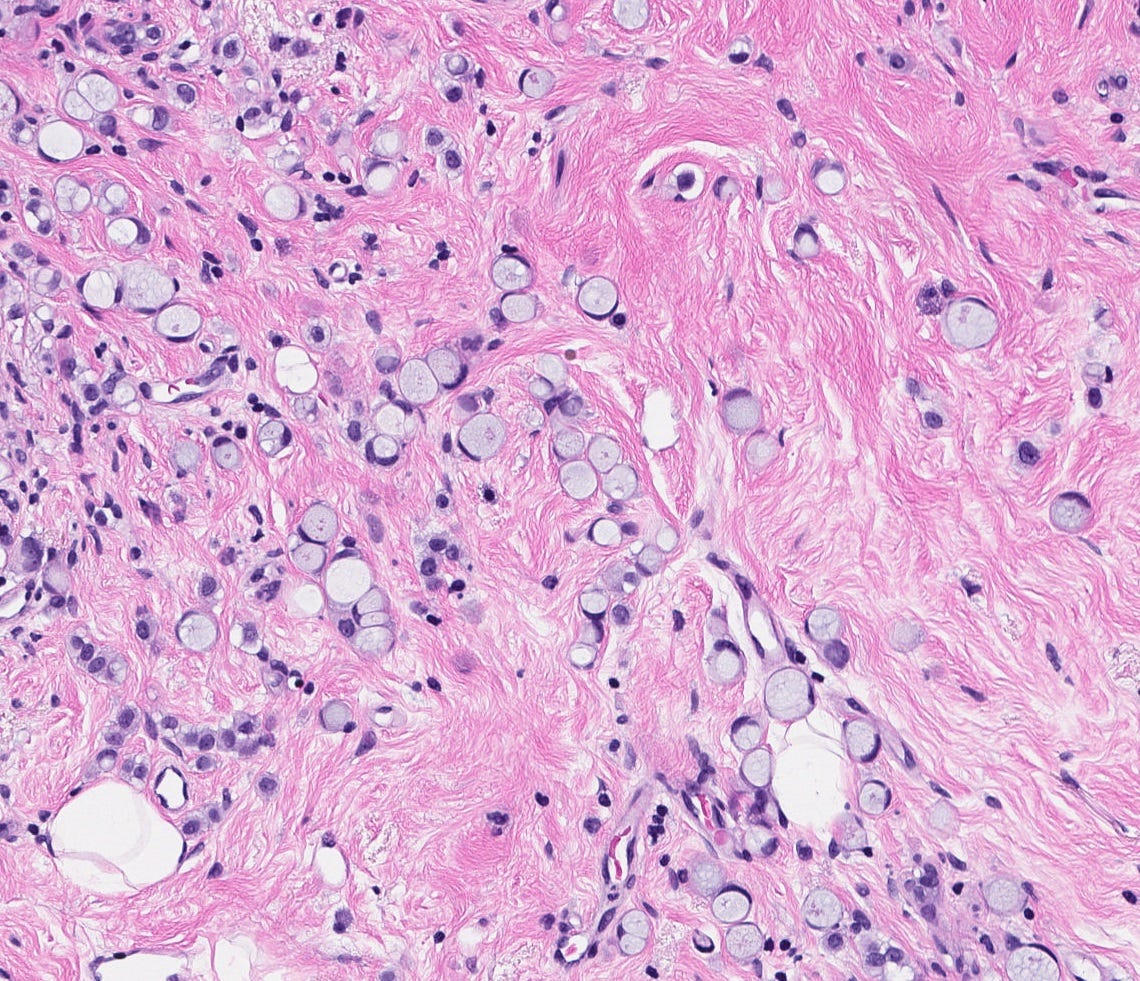
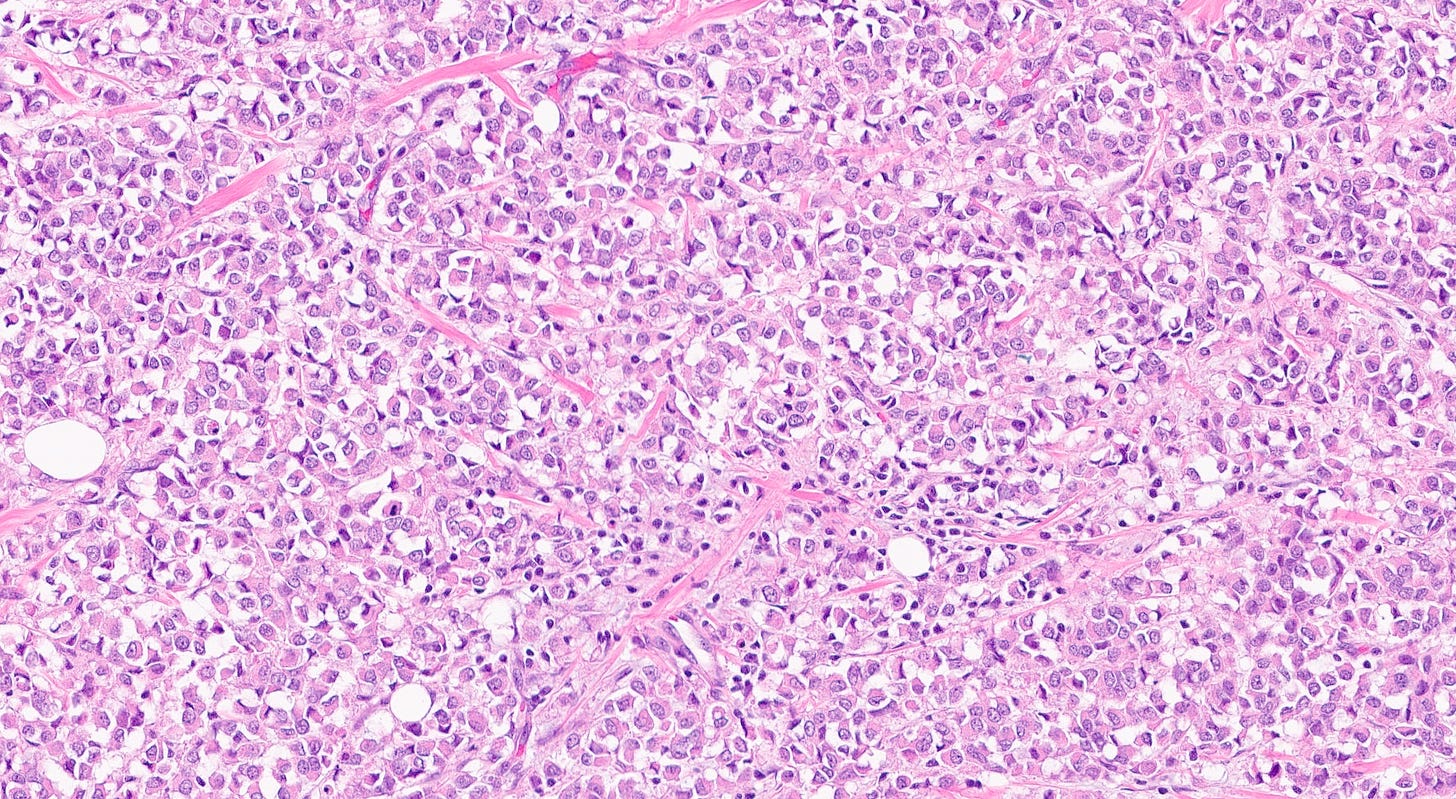
The premalignant precursors of classic lobular carcinoma are classic lobular carcinoma in situ (classic LCIS), its variants florid LCIS and pleomorphic LCIS, and atypical lobular hyperplasia (ALH).
Precursor of classic lobular carcinoma - classic lobular carcinoma in situ
As noted above, classic LCIS, unlike DCIS, is no longer considered a malignant condition. Excision is recommended only if other high risk proliferative lesions are found or there is discordance between histology and imaging.
Precursor of classic lobular carcinoma - florid lobular carcinoma in situ
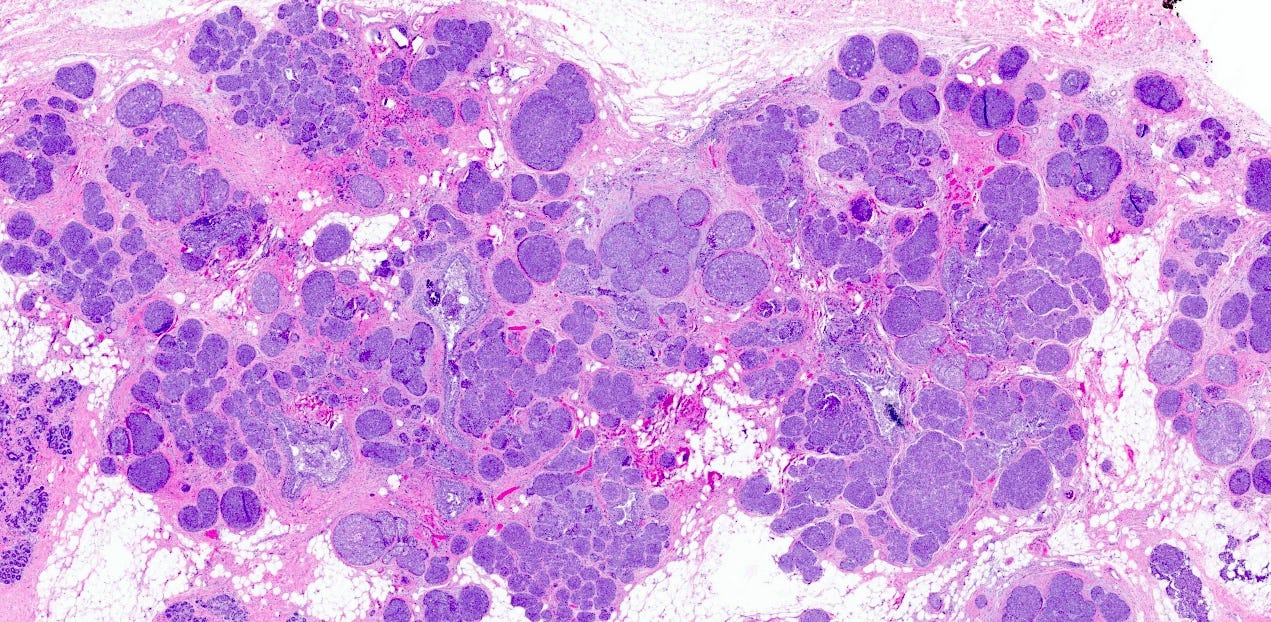
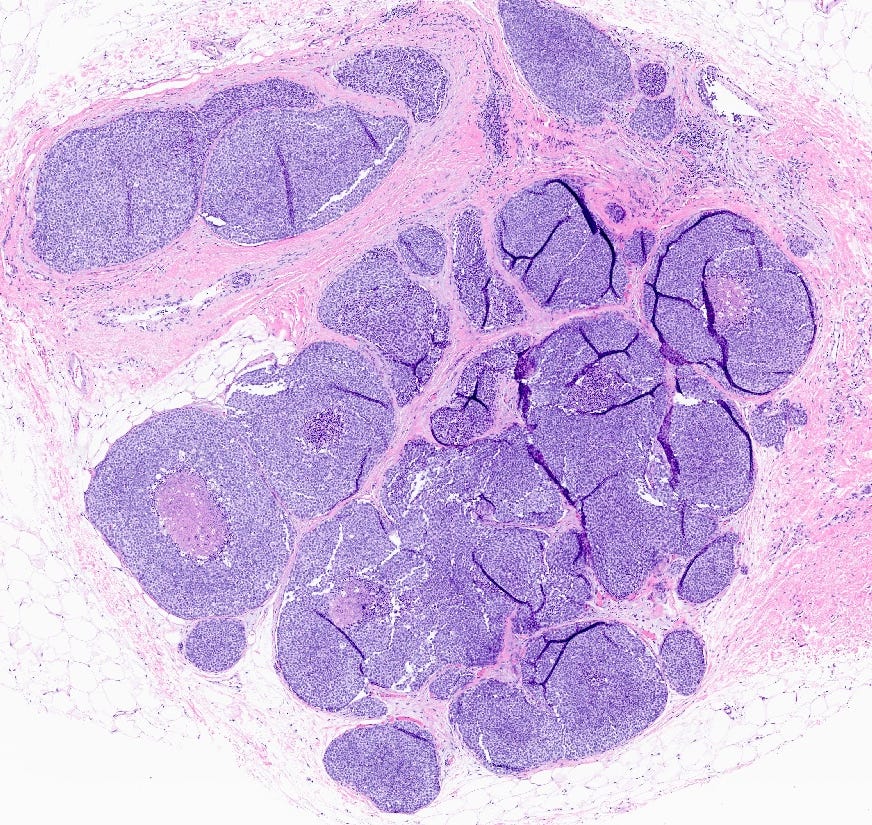
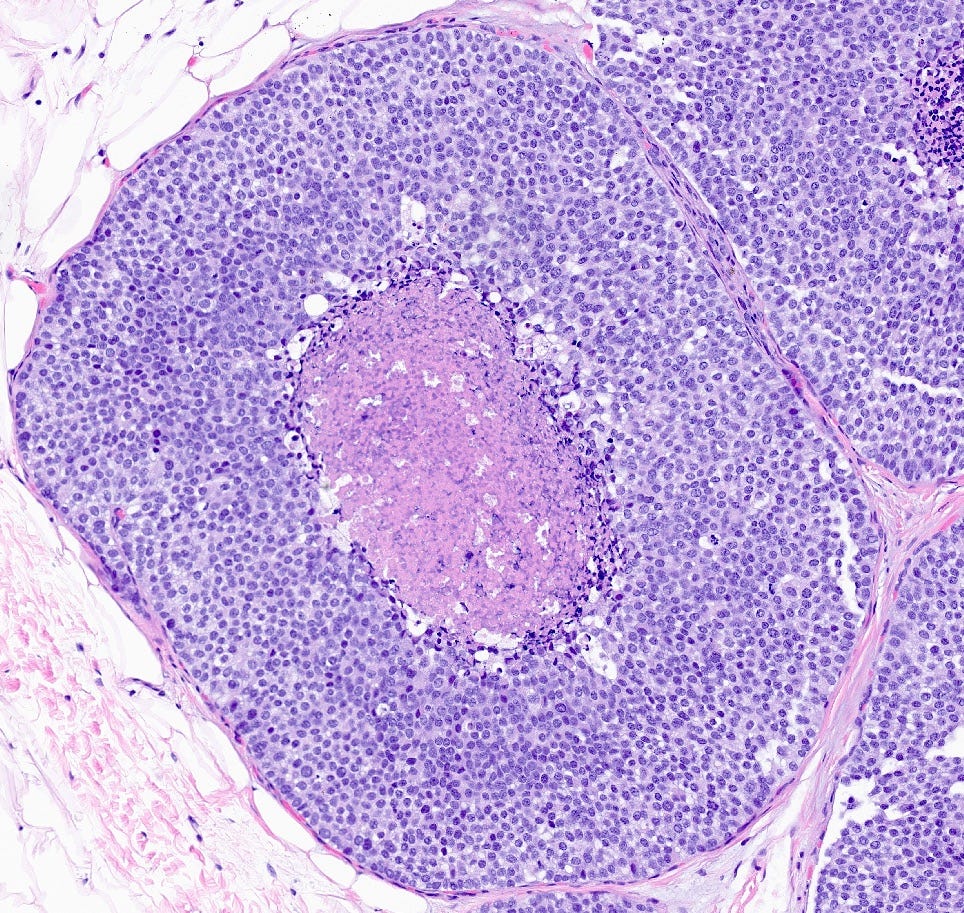
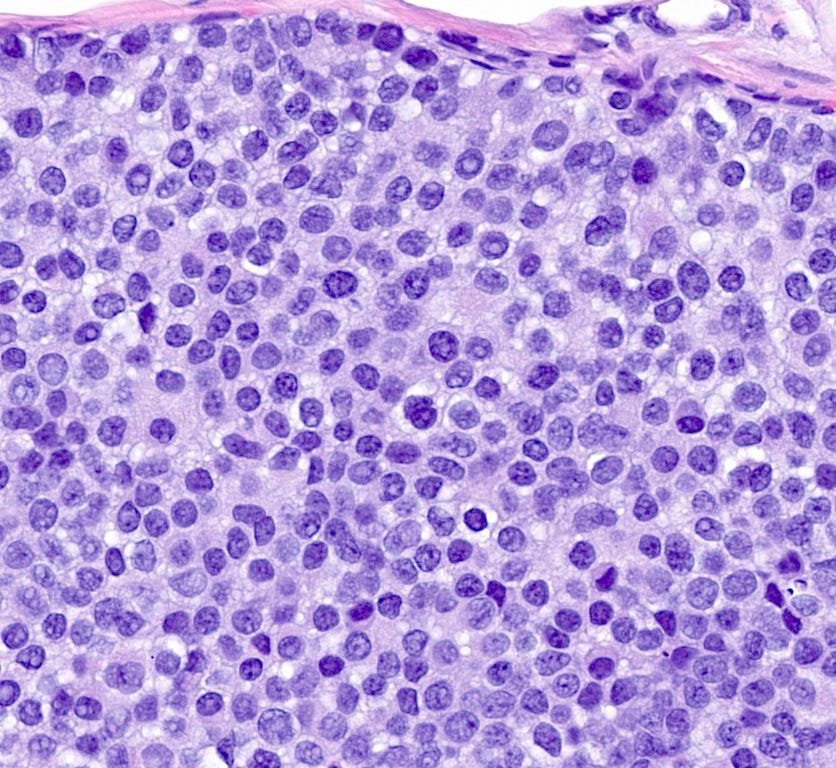
Florid LCIS is a rare (< 5% of LCIS) nonclassical or variant form of lobular carcinoma in situ in which acini and ducts are markedly distended with little to no intervening stroma. It may have a mass-like architecture and includes cells with cytologic features of classic LCIS (type A or type B cells) with or without comedonecrosis and calcifications. Most cases are associated with classic LCIS or atypical lobular hyperplasia (ALH).
Florid LCIS has features of a high risk precursor lesion of invasive carcinoma including a higher upgrade rate, increased association with invasive carcinoma and more genetic complexity compared to classic LCIS.
In contrast to classic LCIS, complete surgical excision of florid LCIS is recommended.
Florid lobular carcinoma in situ - microscopic images
Precursor of classic lobular carcinoma - pleomorphic lobular carcinoma in situ
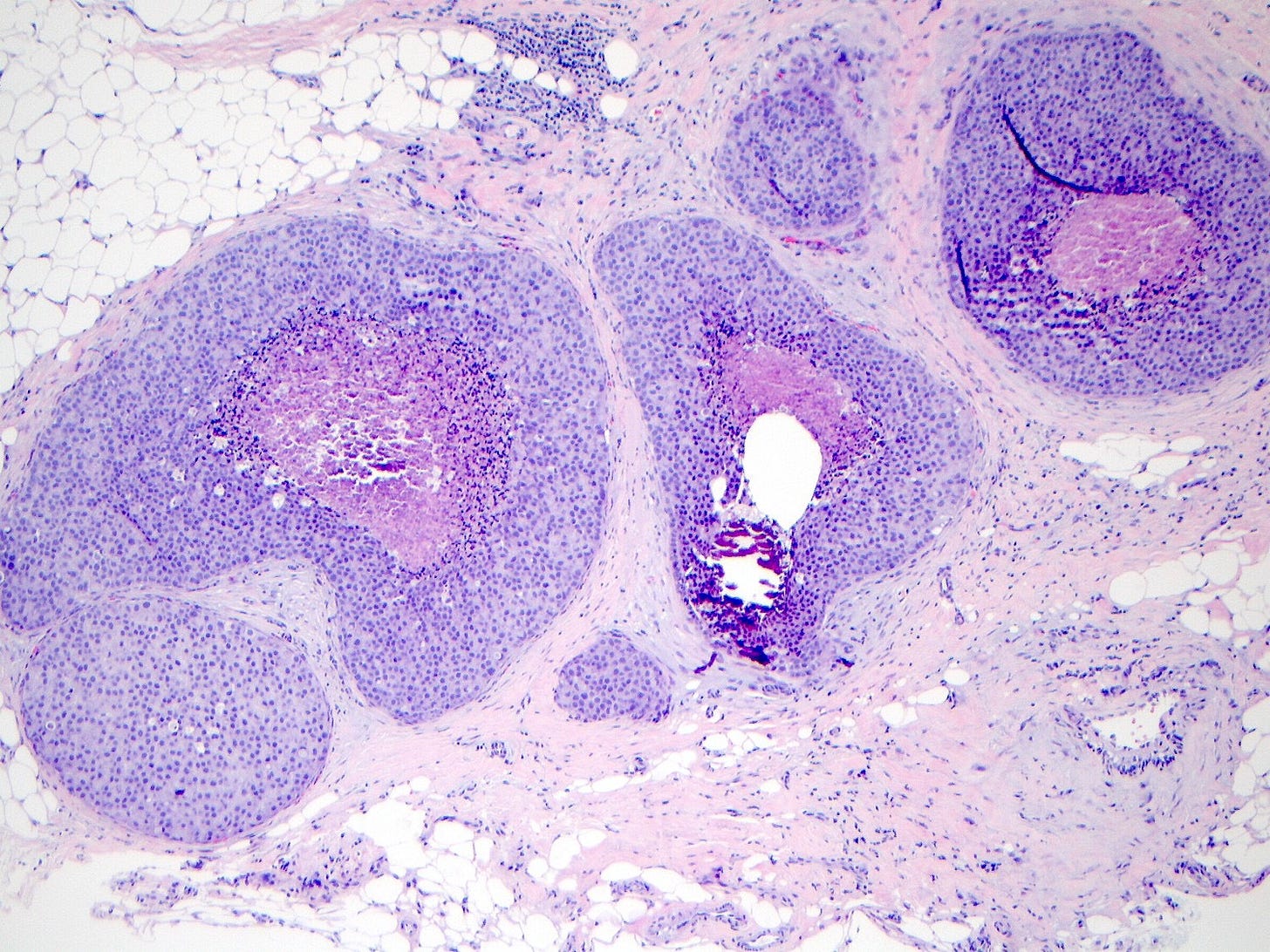
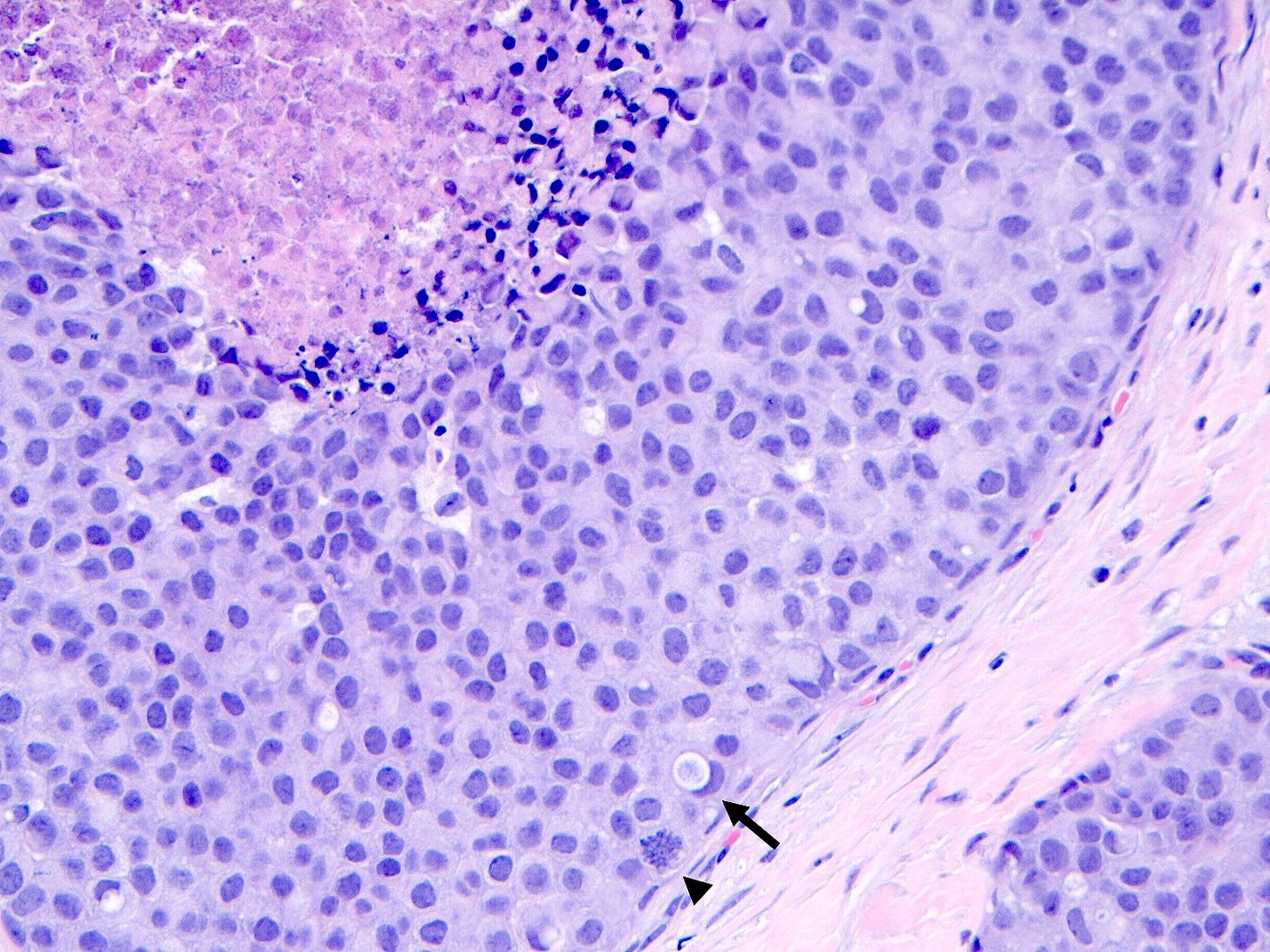
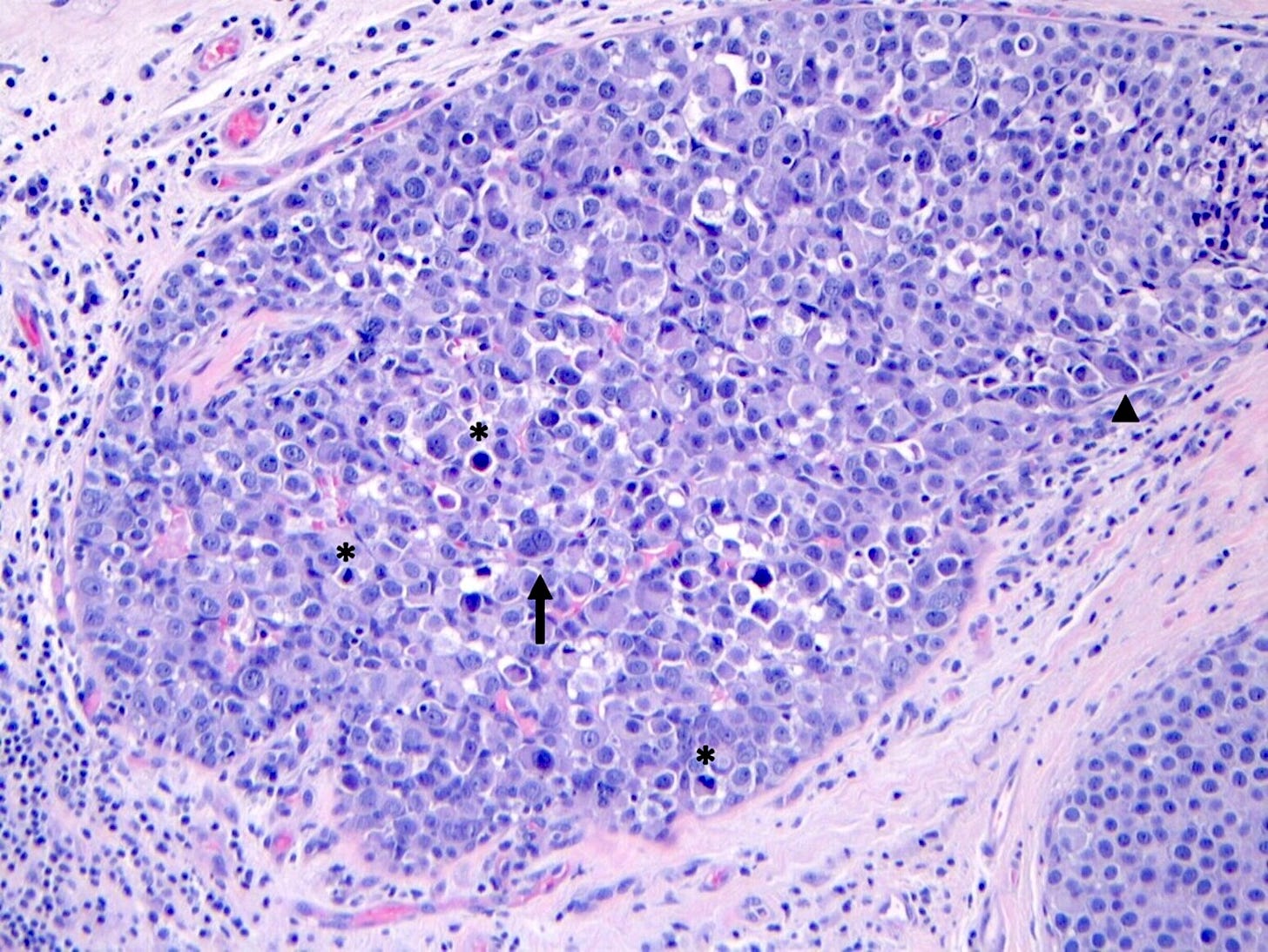
Pleomorphic LCIS is a rare (< 5% of LCIS) nonclassical or variant form of lobular carcinoma in situ composed of a noninvasive, neoplastic proliferation of large dyscohesive cells with marked nuclear pleomorphism. Its nuclei are > 4 times the size of a lymphocyte and its cytologic features more closely resemble high grade DCIS than classic LCIS. It may exhibit comedonecrosis and calcifications. However, other features overlap with classic LCIS: loss or dysfunction of E-cadherin, cellular dyscohesion, lack of cell polarity, intracytoplasmic lumina, signet ring cells and pagetoid spread. Most cases are associated with classic LCIS and ALH.
Immunostains for E-cadherin may assist in distinguishing LCIS from DCIS although they must be interpreted in the context of the histologic features.
Pleomorphic LCIS accumulates additional alterations and generally exhibits greater genomic instability than classic LCIS.
Pleomorphic LCIS has features of a high risk precursor lesion of invasive carcinoma. It is associated with a high incidence (49%) of associated invasive carcinoma either on core needle biopsy or excision. Treatment consists of surgical excision but there is no consensus on requiring negative margins, adjuvant treatments or sentinel node biopsy.
Pleomorphic lobular carcinoma in situ - microscopic images
Precursor of classic lobular carcinoma - atypical lobular hyperplasia
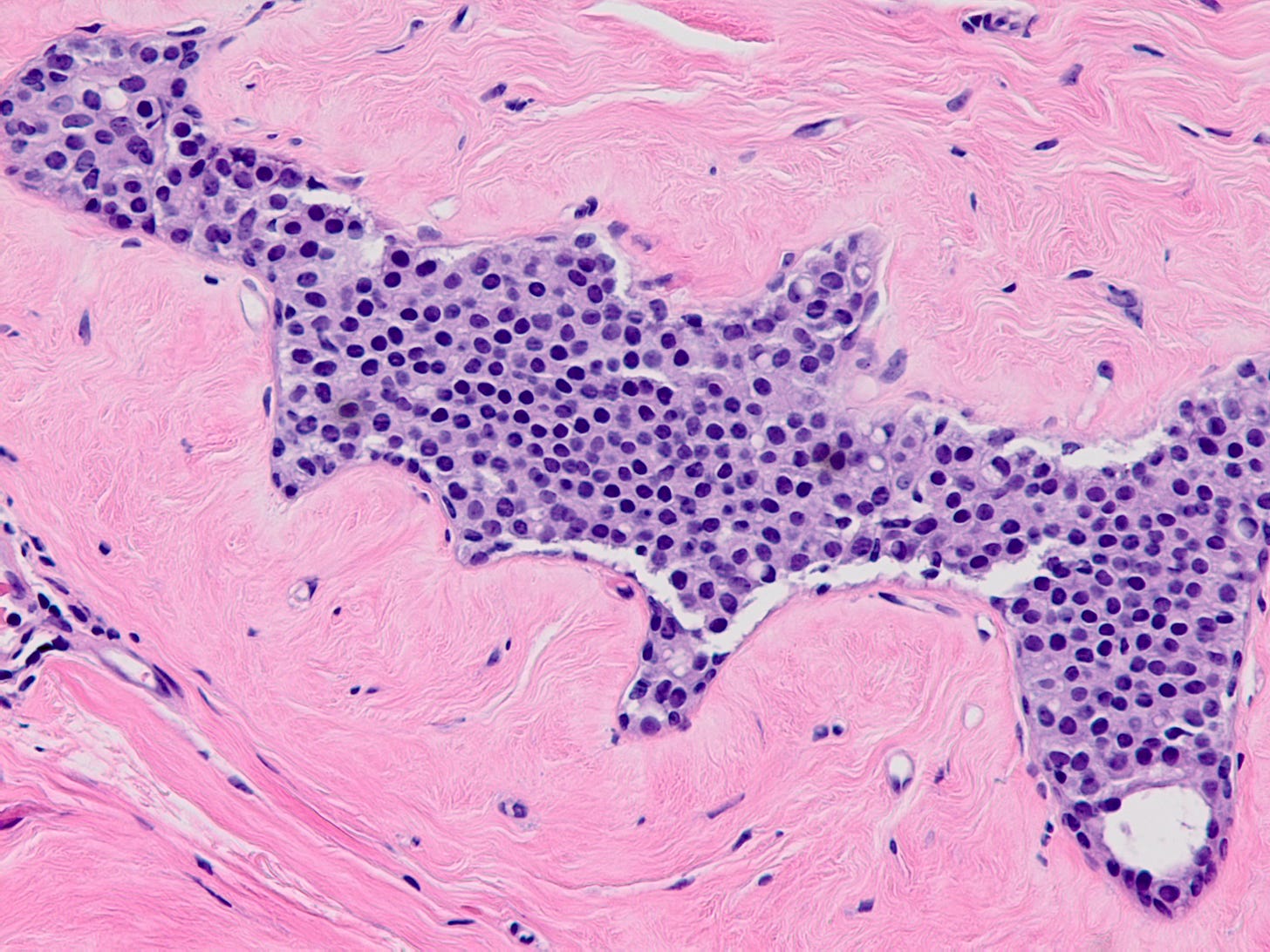
Atypical lobular hyperplasia (ALH) is a clonal proliferation of dyscohesive epithelial cells arising in terminal duct lobular units with similar histologic features as LCIS but of insufficient quantity for that diagnosis. It shows the typical dysfunction or loss of E-cadherin associated with LCIS.
ALH is generally considered an incidental finding. It has a 4 - 5 times increased risk of invasive breast carcinoma compared with the general population. Like classic LCIS, it is often treated conservatively with active surveillance and anti estrogens. Excision may be appropriate if there is radiologic pathologic discordance (suggesting that the targeted lesion was not excised), other high risk lesions are present, it has features resembling DCIS or it has pleomorphic features.
Atypical lobular hyperplasia - microscopic images
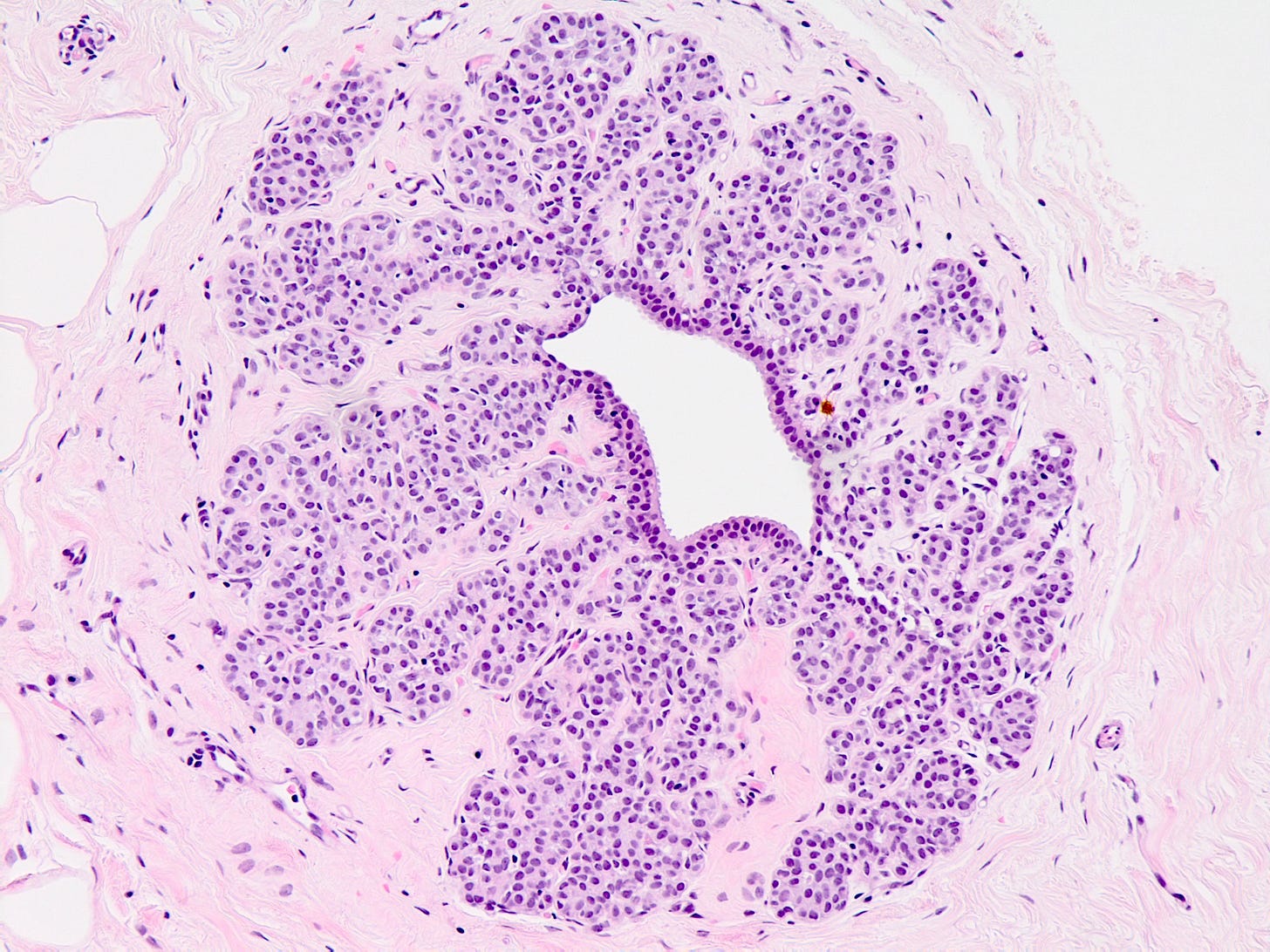
Pleomorphic lobular carcinoma
Pleomorphic lobular carcinoma is a less common subtype of infiltrating lobular carcinoma (15%) with cellular dyscohesion and histologic architecture similar to classic infiltrating lobular carcinoma plus pleomorphic cytologic features similar to those seen in pleomorphic LCIS or high grade DCIS. Its molecular profile and immunostaining pattern (including the loss of E-cadherin) support a common origin with infiltrating lobular carcinoma with the acquisition of further molecular alterations.
It typically occurs in postmenopausal women ages 60 - 80 and is more commonly represented in BRCA2 carriers, in whom it presents at a much younger age.
Most patients present with a poorly defined palpable breast mass. Patients typically have a more advanced stage (large tumors and axillary lymph node metastasis) than patients with invasive ductal carcinoma (IDC). The cancer is more frequently multifocal or multicentric.
Treatment consists of surgical resection (conservative for local disease, mastectomy if multifocal) and adjuvant therapy (usually endocrine therapy, possibly anti-HER2 therapy if appropriate.
Its prognosis is similar to that of other high grade breast carcinomas of similar stage or grade, although some studies suggest its histology is associated with poorer survival.
Pleomorphic lobular carcinoma - microscopic images
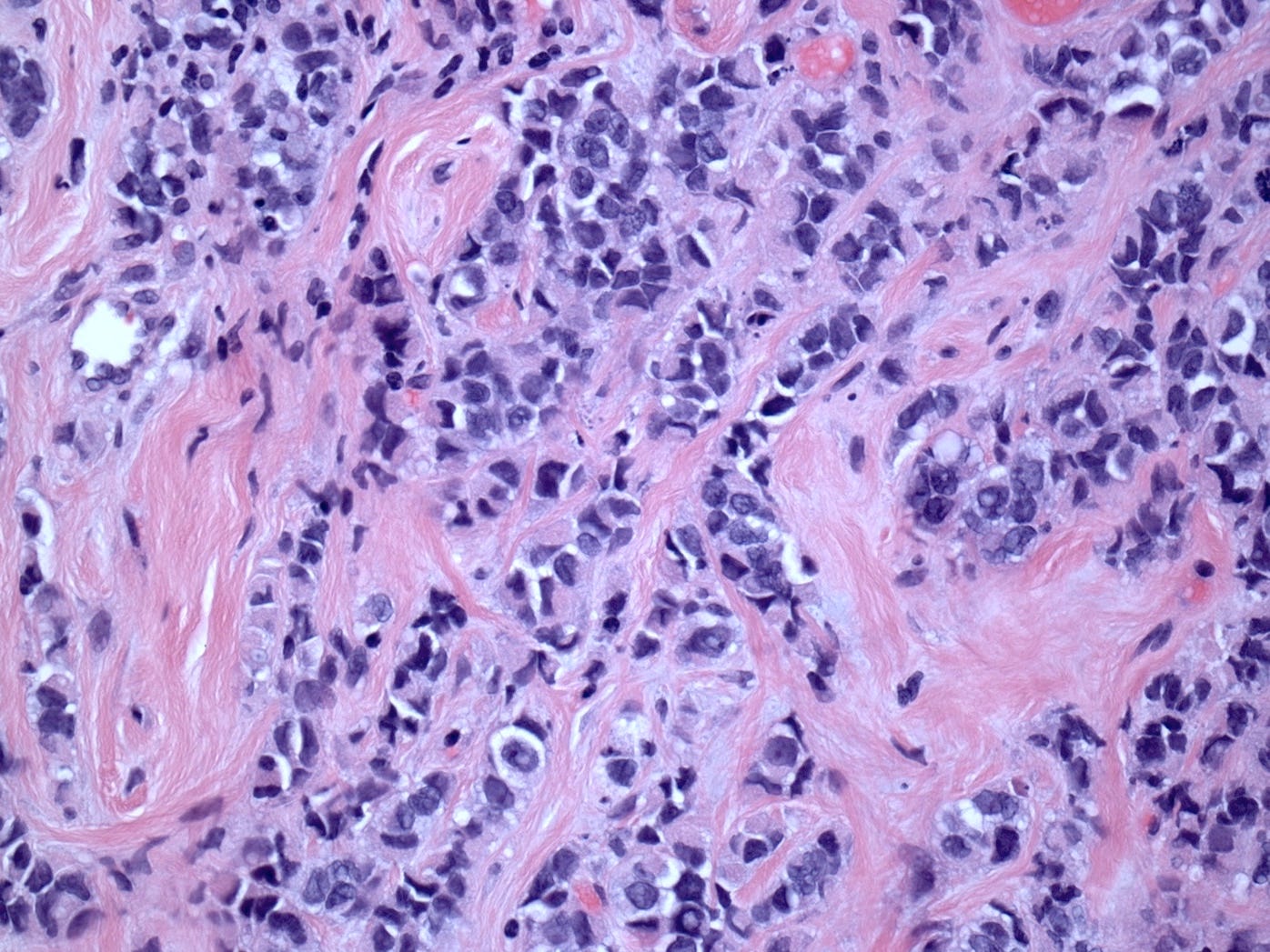
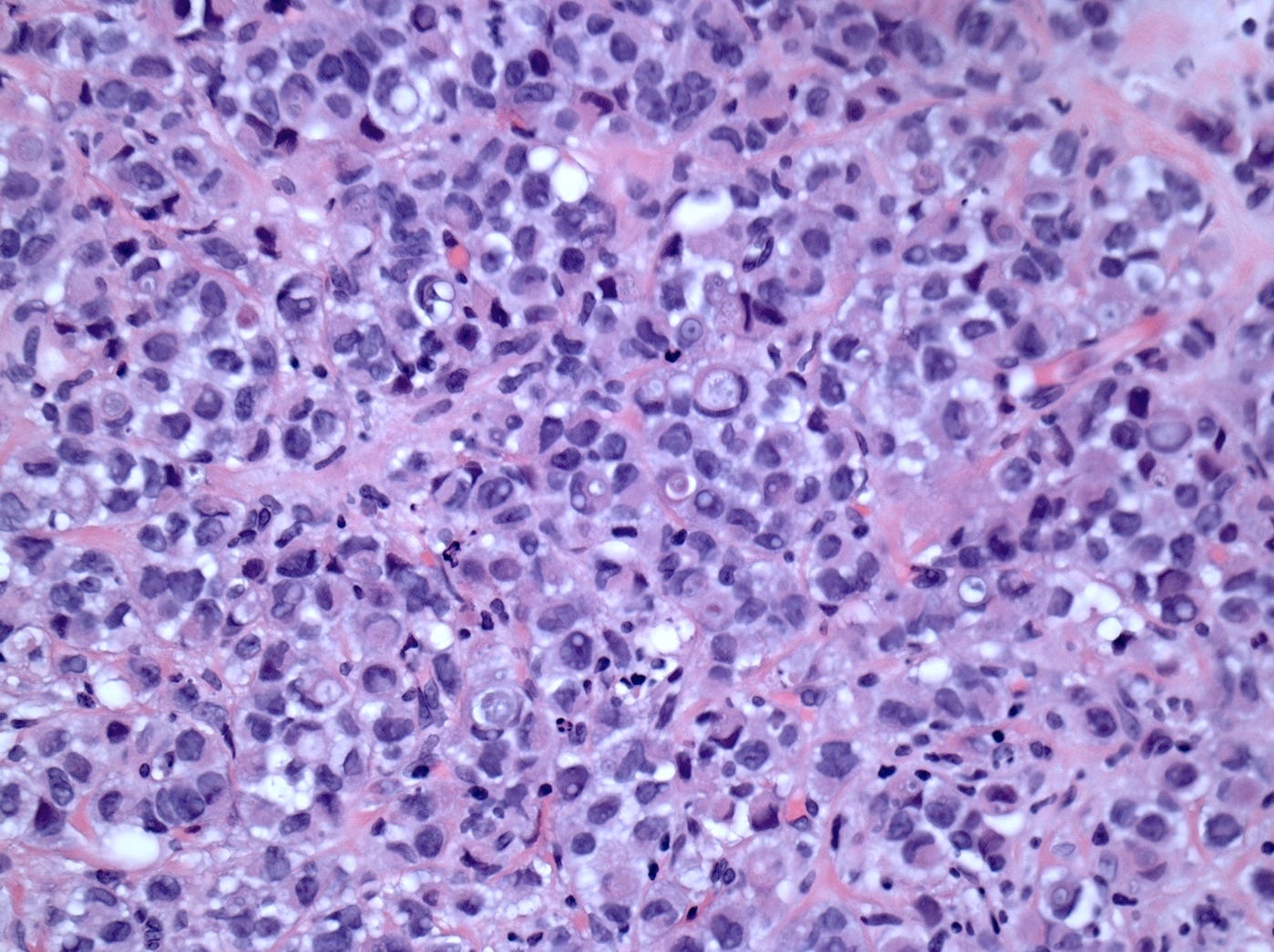
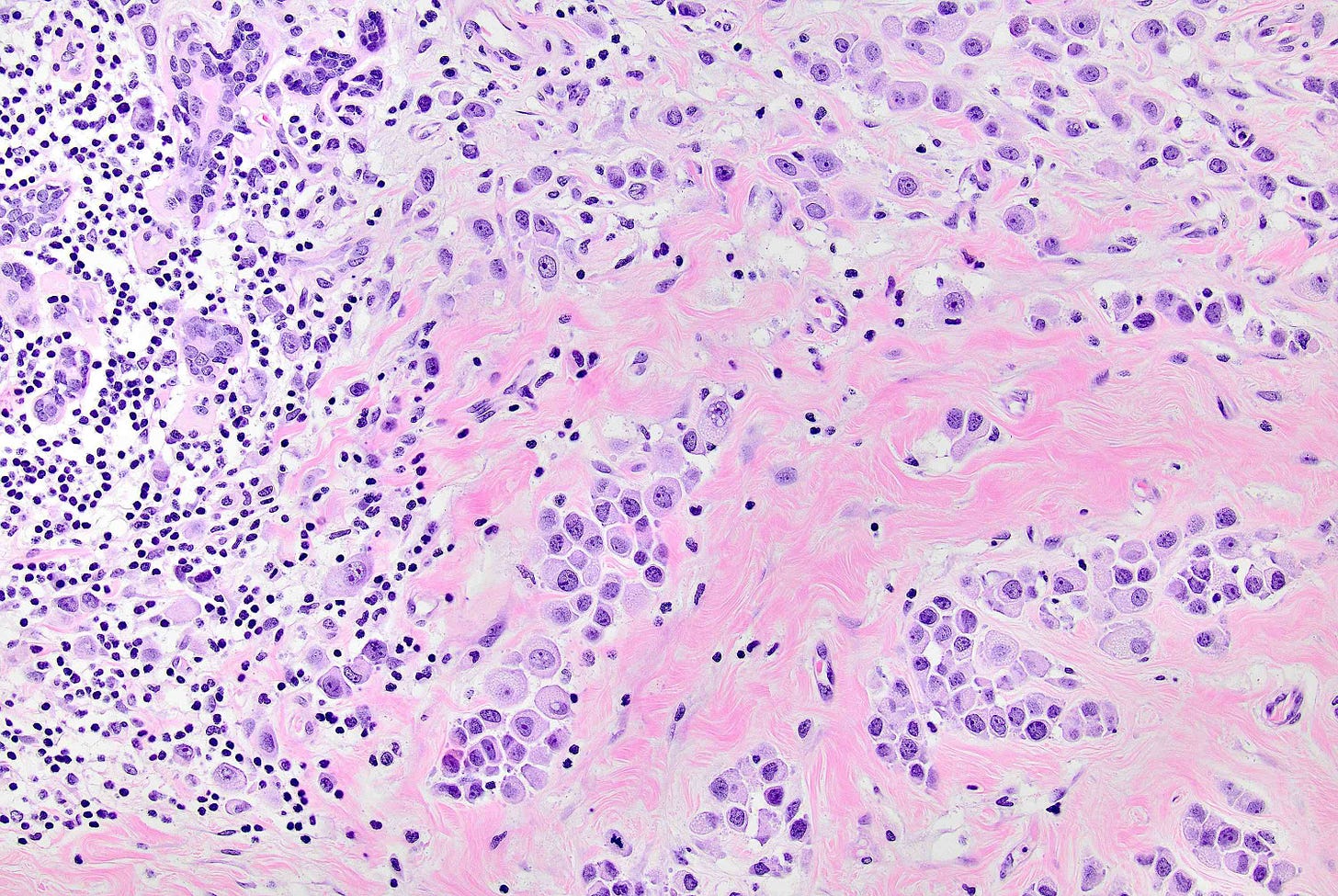
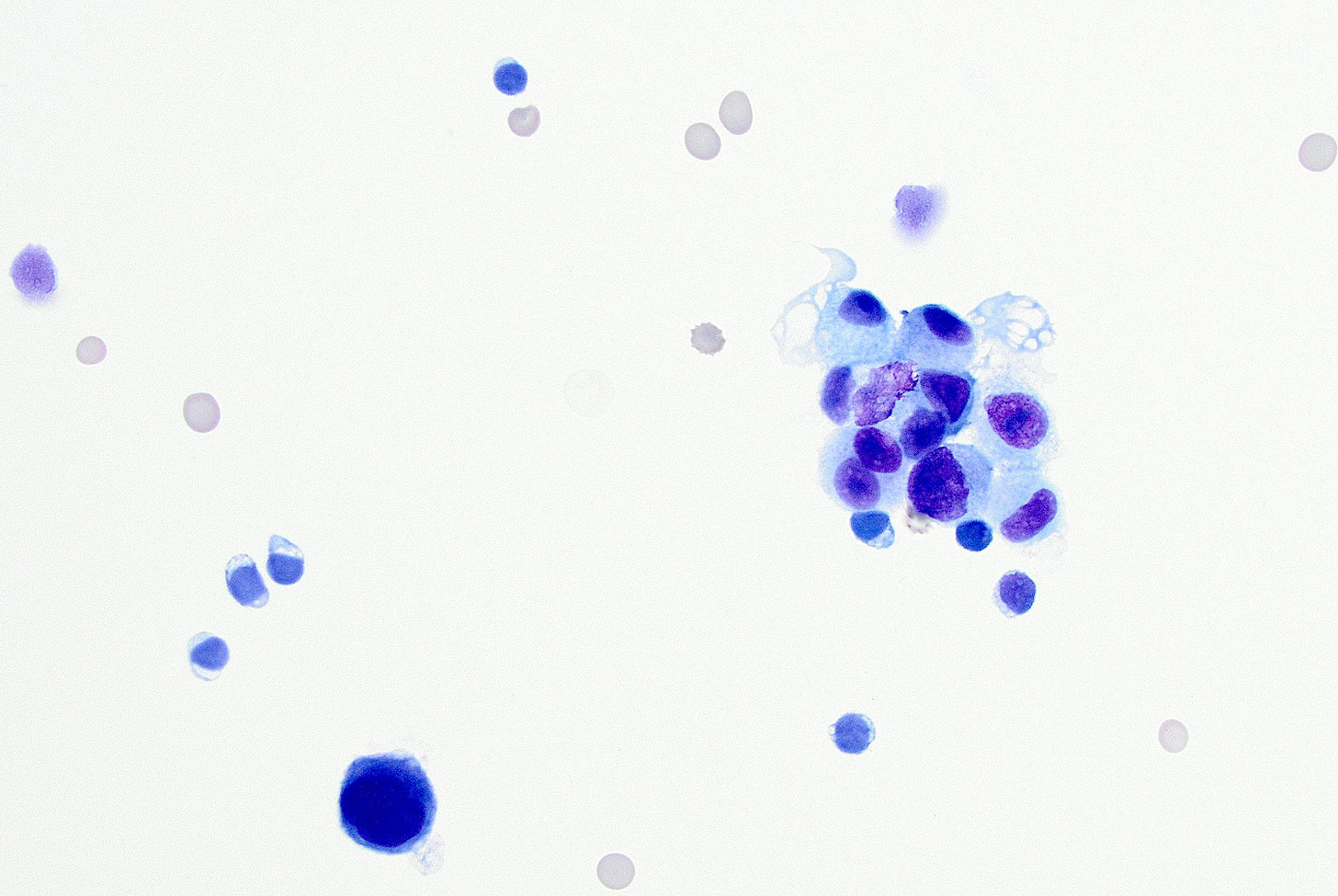
The precursors of pleomorphic lobular carcinoma are pleomorphic lobular carcinoma in situ and classic LCIS, which frequently coexist. They were discussed previously.
The next essay will discuss additional breast malignancies with known precursors.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me at https://www.linkedin.com/in/nat-pernick-8967765/ (LinkedIn), npernickmich (Threads and Instagram), natpernick.bsky.social (Bluesky) or @nat385440b (Tribel).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week we post interesting cancer related images of malignancies with diagnoses plus articles of interest. Please also read our CCN essays.
Latest versions of our cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email me at Nat@PathologyOutlines.com - Unfortunately, I cannot provide medical advice.
I also publish Notes at https://substack.com/note. Subscribers will automatically see my Notes.





We removed a comment, which was basically an advertisement.