Cancer precursor project - Neuroendocrine tumors
12 March 2024
This is our first essay discussing malignancies and their precursor lesions based on our new Cancer Precursor Project. We have compiled a spreadsheet of all distinct human cancers (~1230) and their identifiable precursors (~180). Please email proposed updates to Nat@PathologyOutlines.com.
Our goal is to identify precursor lesions for all malignancies to better understand and treat cancer and reduce its 600,000 annual U.S. deaths. Studying known precursors and their patterns of molecular expression may suggest molecular patterns for tumors with unknown precursors (Pernick 2018).
This essay discusses data compiled by our project on neuroendocrine tumors and summarizes current knowledge about two precursor lesions, one in the stomach (enterochromaffin-like cell hyperplasia) and one in the lung (diffuse idiopathic pulmonary neuroendocrine hyperplasia).
Neuroendocrine neoplasms are typically divided into neuroendocrine tumors-well differentiated (NET), neuroendocrine carcinomas- poorly differentiated (NEC) and mixed neuroendocrine-non-neuroendocrine neoplasms (MINEN). Neuroendocrine tumors are graded as G1, G2, or G3 (G = grade) based on proliferative activity or mitotic rate and Ki-67 labeling (Biancotti 2023):
Classification of gastrointestinal well differentiated neuroendocrine tumors
![Classification of NETs of gastro-entero-pancreatic tract [1] Classification of NETs of gastro-entero-pancreatic tract [1]](https://substackcdn.com/image/fetch/$s_!O40J!,w_1456,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F596a224e-6ec3-41f1-b959-63fc56bdc239_499x190.png)
This essay discusses neuroendocrine tumors (NET), which are malignant because they have aggressive or invasive properties at least occasionally. We do not discuss other malignant neuroendocrine neoplasms: neuroendocrine carcinomas (small cell and large cell subtypes), neuroendocrine tumors with sarcomatous differentiation, mixed neuroendocrine-non-neuroendocrine neoplasms, neuroblastoma (adrenal, CNS and olfactory), medullary thyroid carcinoma and Merkel cell carcinoma. Links are to PathologyOutlines.com topics if available, otherwise to PubMed references.
Neuroendocrine tumors are found throughout the body, including at all nine gastrointestinal sites:
Pancreas (general)
There are specific designations for pancreatic neuroendocrine tumors based on whether they are nonfunctioning or functioning:
These are the nonfunctioning pancreatic neuroendocrine tumors:
These are the functioning pancreatic neuroendocrine tumors:
Enterochromaffin cell carcinoid - no references identified
At non-gastrointestinal sites, we have identified these neuroendocrine tumors as distinct malignant entities:
Most articles discussing precursors for neuroendocrine tumors appear to assume that they are the same for G1, G2 and G3 tumors. Historically it was assumed that malignant transformation is a stepwise process from premalignant to low grade to intermediate grade and then to high grade lesions. However, this may not be correct due to the presence of multiple parallel, genetically distinct pathways (Tang 2006); some high grade lesions seem to start that way and do not represent transformations of lower grade lesions. Neuroendocrine tumors are not a precursor for neuroendocrine carcinomas.
Precursor lesions for neuroendocrine tumors - stomach
Stomach neuroendocrine tumors are classified based not only on tumor grade (proliferative activity) but on how they arise. Although precursor lesions have been identified for some types, as indicated below, it is not clear whether they are the same for types 1, 2 or 3 lesions. Most GI neuroendocrine tumors have no known precursors.
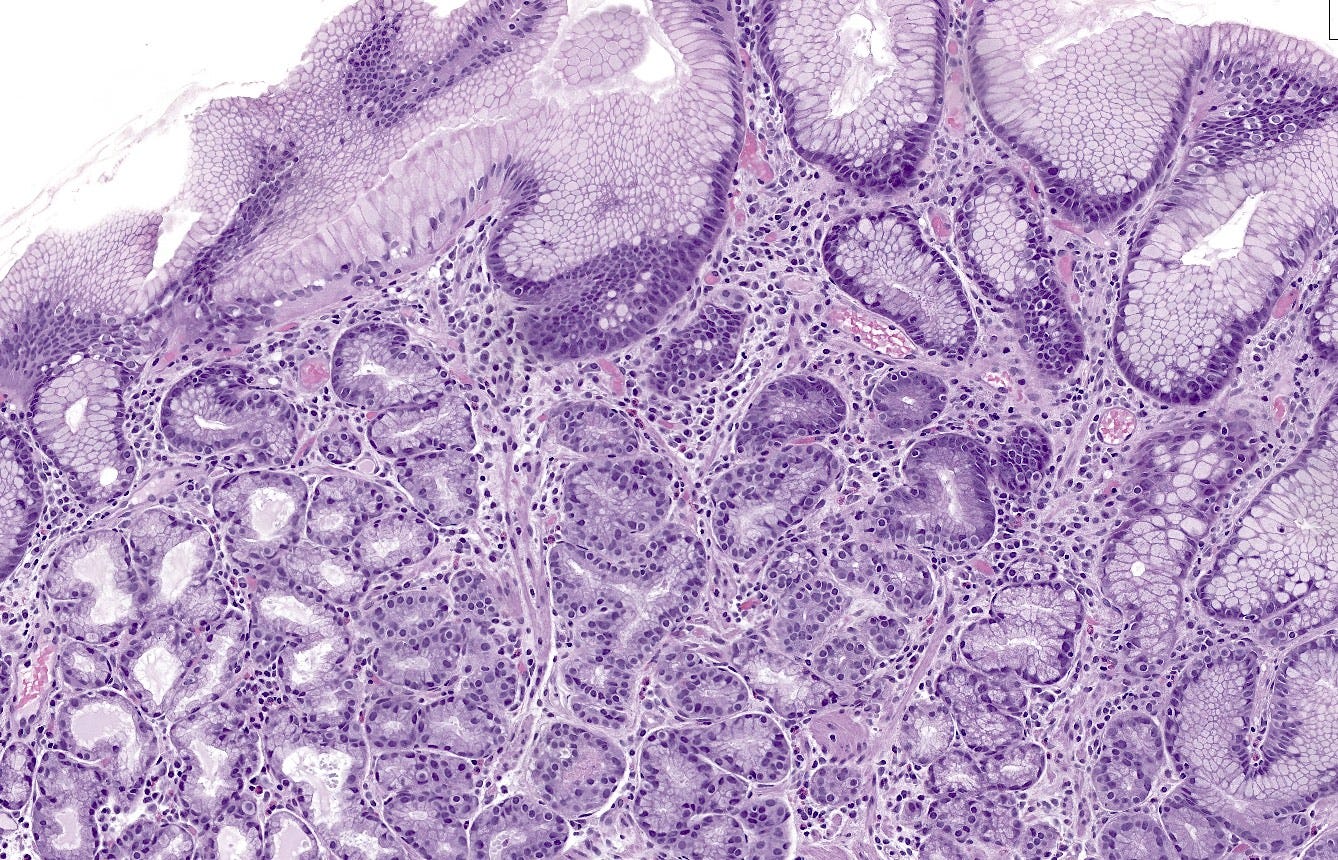
Type 1 neuroendocrine tumors in the stomach are typically caused by atrophic gastritis (a chronic inflammatory disease due to Helicobacter pylori infection) (Massironi 2023) or autoimmune gastritis (destruction of gastric glands that normally produce acid by autoantibodies). Decreased acid production causes compensatory G (gastrin) cell hyperplasia (particularly in autoimmune gastritis), resulting in gastrin overproduction (hypergastrinemia), which stimulates hyperplasia of enterochromaffin-like cells (ECL cells), which is a precursor lesion for neuroendocrine tumors (Vanoli 2013), both grade 1 (Poveda 2023) and perhaps some grade 2 tumors (Yu 2022). To our knowledge, precursors to grade 3 tumors have not been described. It is unclear at what point hyperplastic lesions are considered to become a precursor for a neuroendocrine tumor.
Type 2 tumors also arise in the setting of hypergastrinemia but this is due to a gastrin secreting tumor (gastrinoma) causing Zollinger-Ellison syndrome (Gonzalez 2020). The gastrinoma itself may be sporadic in the pancreas or duodenum or occur as part of multiple endocrine neoplasia type 1 (MEN1) (Mete 2013). Excess gastrin levels may cause enterochromaffin-like cell hyperplasia, perhaps because the MEN1 mutation sensitizes ECL cells to the mitogenic effect of gastrin (Berna 2008). Cases of enterochromaffin-like cell hyperplasia with “severe linear hyperplasia” may be a precursor lesion for type 2 tumors (Biancotti 2023).
Type 3 tumors have an unknown etiology and no known precursor. They are not associated with hypergastrinemia, atrophic gastritis or enterochromaffin-like cell hyperplasia (Biancotti 2023).
Type 4 tumors are associated with hypergastrinemia and parietal cell hyperplasia but without MEN1 or Zollinger-Ellison syndrome (Biancotti 2023, Abraham 2005, Ooi 1995). They may be due to a defect in the molecular protein pump (genetic or acquired) (Shiroma 2022). There is no known precursor.
Type 5 tumors develop in patients with hypergastrinemia caused by proton pump inhibitor use for at least one year, with no evidence of atrophic gastritis, gastrinoma or MEN1 (Rais 2022). Most of these patients do not develop neuroendocrine tumors despite persistent hypergastrinemia, suggesting that chronic hypergastrinemia by itself is insufficient for the development of gastric neuroendocrine tumors (Biancotti 2023).
Whether other neuroendocrine tumors are due to endocrine cell hyperplasia needs to be studied.
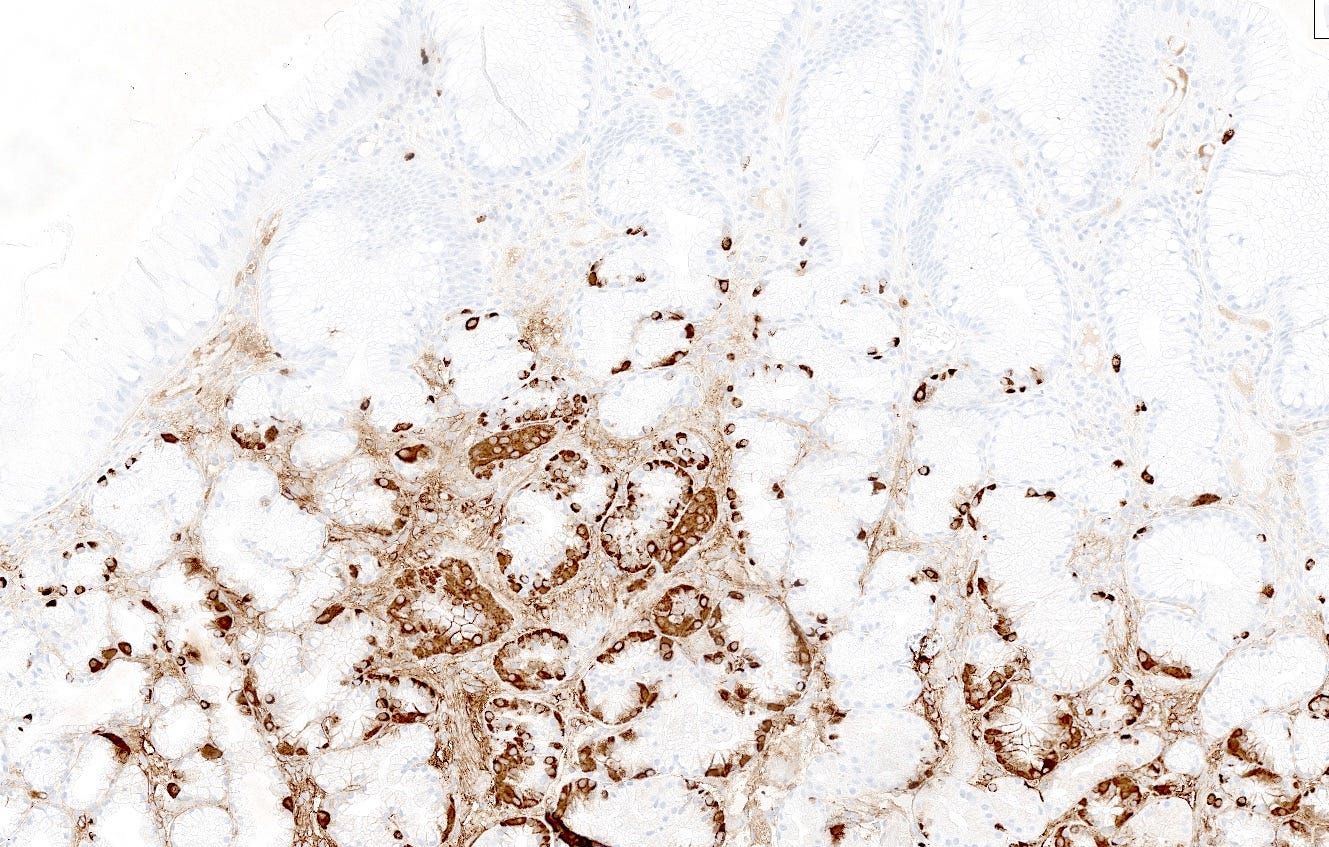

Precursor lesions to neuroendocrine tumors - lung
In the lung, diffuse idiopathic pulmonary neuroendocrine hyperplasia (DIPNECH) is considered a precursor lesion that may progress to tumorlets or carcinoid tumors, usually typical carcinoid (grade 1) but also possibly atypical carcinoid (grade 2, Takegahara 2017). In primary DIPNECH, the cause of the hyperplasia is unknown and must be investigated. Microscopically, there is a generalized intramucosal proliferation of pulmonary neuroendocrine cells in monolayers or small groups that can penetrate through the bronchial basement membrane to form tumorlets. However, this condition is difficult to diagnose due to the lack of validated diagnostic criteria. It must be differentiated from secondary DIPNECH, a localized neuroendocrine proliferation secondary to another chronic lung disease which often is not considered a precursor lesion.
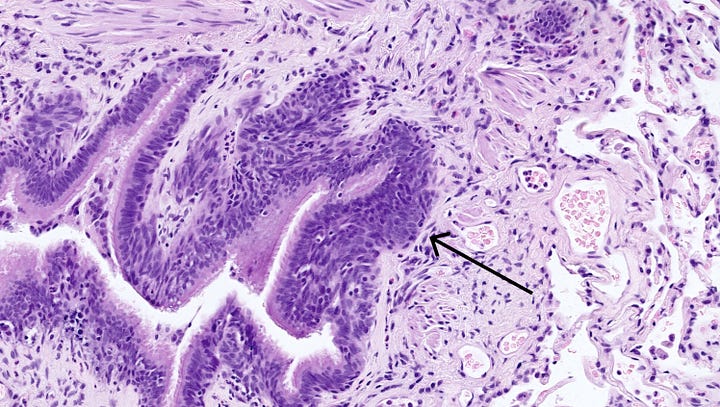

Left: proliferating neuroendocrine cells form small nodules (arrow) limited to the bronchiole. Right: a discrete proliferation of neuroendocrine cells in a linear form (arrows) and limited to the bronchiole. Contributed by Andréanne Gagné, M.D., M.Sc. and Philippe Joubert, M.D., Ph.D.
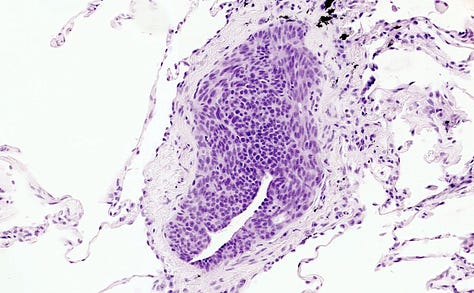

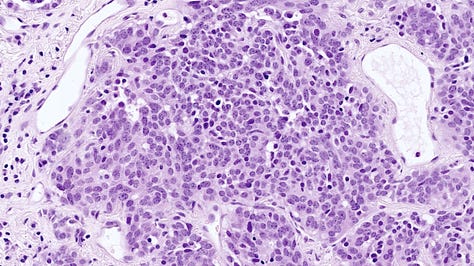
Left: proliferating neuroendocrine cells form a nodule occluding a small bronchiole. Middle: proliferating neuroendocrine cells in the bronchial mucosa (red arrow) cross beyond the mucosal basal lamina to form a tumorlet (black arrow). Right: a tumorlet showing neuroendocrine cells with salt and pepper chromatin with inconspicuous nucleoli. Contributed by Andréanne Gagné, M.D., M.Sc. and Philippe Joubert, M.D., Ph.D.

Future essays will discuss other types of tumors and give more information on the molecular patterns of biomarkers that may be important in identifying precursor lesions.
If you like these essays, please share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine
Follow us on Substack, LinkedIn, Threads and Instagram (npernickmich) and Tribel (@nat385440b).essays
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or Twitter or the CCN section of our PathologyOutlines.com blog. Each week we post interesting cancer related images of malignancies with diagnoses plus articles of interest. Please also read our CCN essays.
Latest versions of our cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email me at Nat@PathologyOutlines.com - Unfortunately, I cannot provide medical advice.
I also publish Notes at https://substack.com/note. Subscribers will automatically see my notes.