This weekly medical blog is dedicated to science, not politics. For political commentary, I maintain a separate daily blog. You're welcome to skip any essays or sections that don’t interest you - no offense taken.
This post is part of my ongoing cancer precursor project, which explores how various cancers develop. Part 6 focuses on breast cancer. You can click here for links to essays on all 45 types of breast cancer (some are still in progress).
In this essay, I examine four forms of triple negative breast cancer: medullary carcinoma, as well as three head and neck tumor types that rarely appear in the breast - adenoid cystic carcinoma, polymorphous adenocarcinoma and tall cell carcinoma with reverse polarity.
Medullary carcinoma of the breast
Medullary carcinoma of the breast is a rare subtype (3 - 5%) of invasive breast cancer with pushing borders, syncytial growth, high grade nuclei and a prominent lymphoid infiltrate. According to the World Health Organization, the preferred terminology is invasive breast carcinoma of no special type with medullary pattern.
Clinically, medullary carcinoma has a better overall and disease free survival than other forms of poorly differentiated invasive ductal carcinoma, not otherwise specified. Lymph node involvement is less common, and distant metastases are relatively infrequent, especially when the tumor is diagnosed and treated at an early stage.
Treatment of medullary carcinoma is identical to that for invasive breast carcinoma of no special type. It usually consists of wide local excision or mastectomy with clear margins, often followed by adjuvant chemotherapy. Sentinel lymph node biopsy is standard for staging purposes, although extensive axillary lymph node dissection is generally reserved for cases with documented nodal involvement. Radiation therapy is typically recommended after breast conserving surgery to reduce the risk of local recurrence.
Grossly, the tumors are usually 2 - 5 cm, well circumscribed, soft to firm, lobulated masses. Although they appear sharply demarcated from surrounding tissue, they lack a true capsule and may exhibit pushing, expansile borders without widespread stromal invasion.
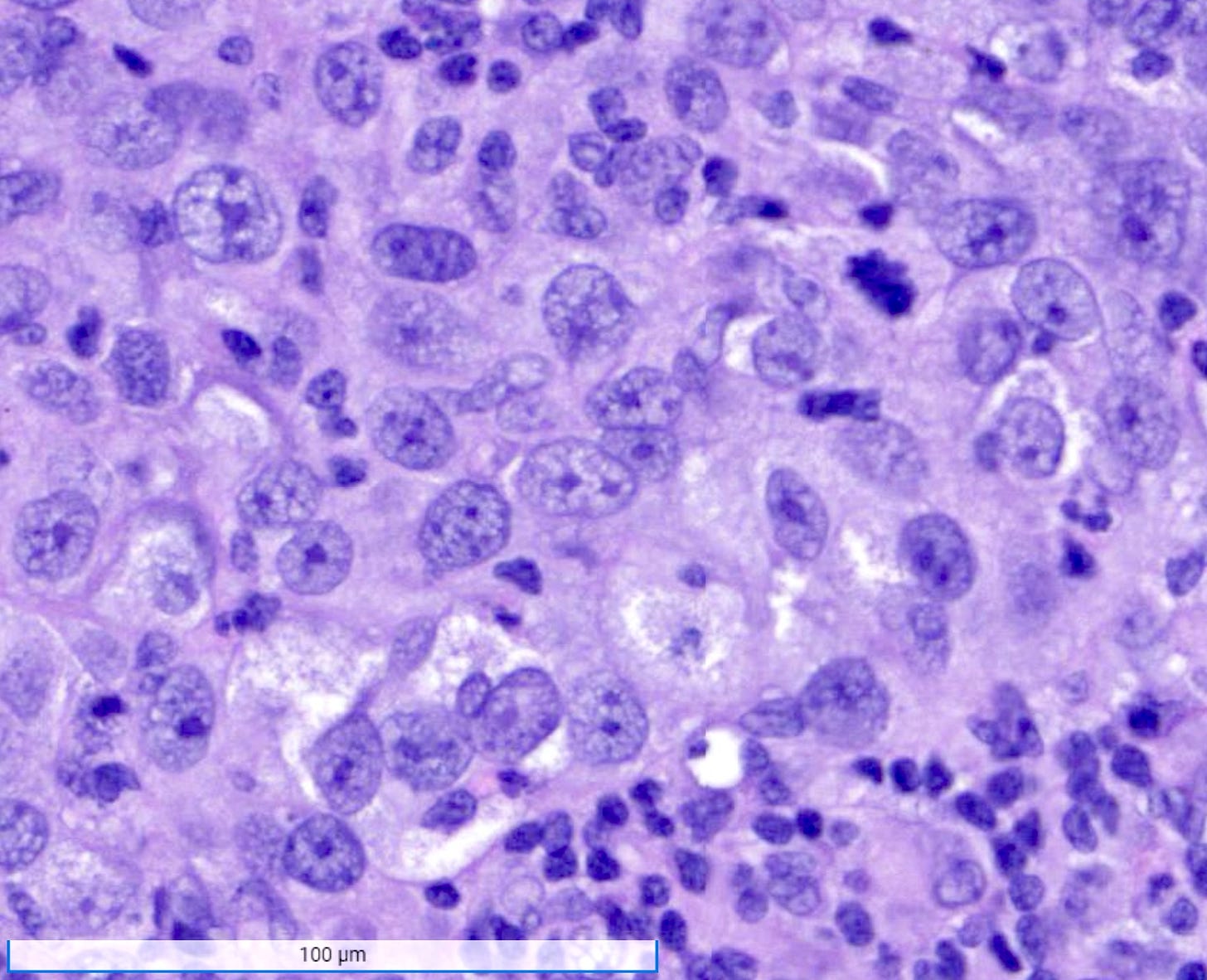
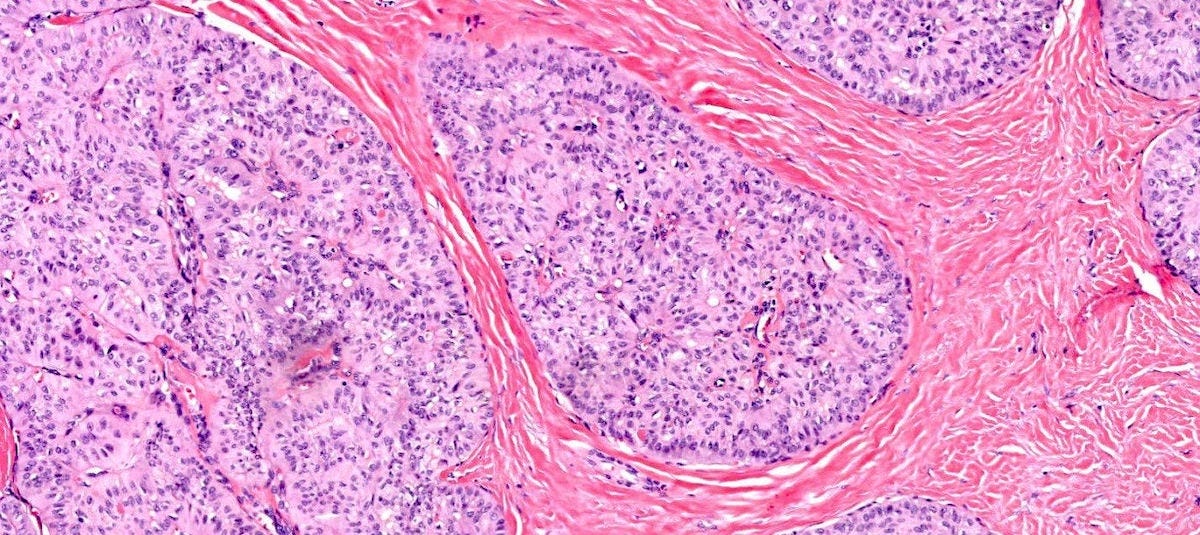
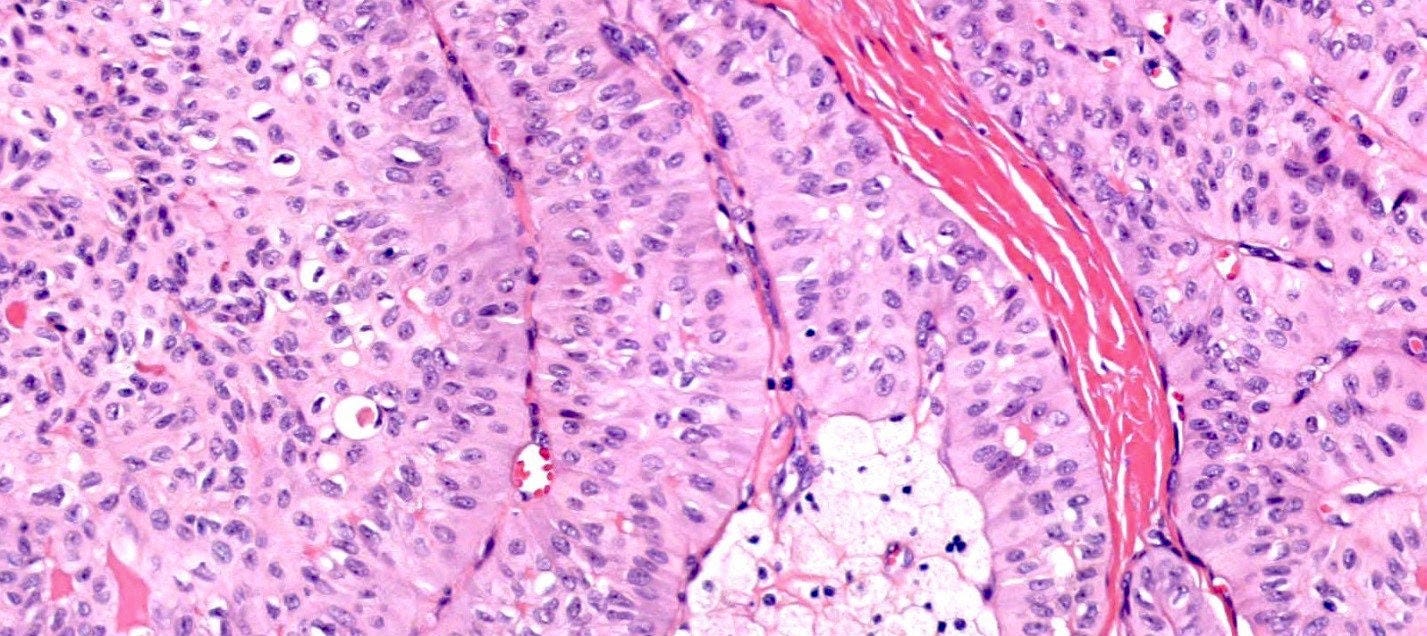
Microscopically, medullary carcinoma is composed of large, pleomorphic, high grade tumor cells arranged in broad syncytial sheets occupying at least 75% of the tumor area. The tumor cells have vesicular nuclei (i.e., large, pale staining with a central clearing) with prominent nucleoli and abundant eosinophilic cytoplasm. A prominent and dense lymphoplasmacytic infiltrate surrounds and often infiltrates the tumor, suggesting a vigorous host immune response. Mitotic figures are numerous and areas of necrosis may be present.
Immunohistochemically, medullary carcinomas typically show a triple negative phenotype (i.e., negative for ER, PR and HER2). However, they frequently express basal cytokeratins such as CK5/6 and epidermal growth factor receptor (EGFR), aligning them with the basal-like molecular subtype. Molecular studies reveal frequent TP53 mutations and a strong association with germline BRCA1 mutations, particularly in patients under 50 years of age or those with a family history of breast or ovarian cancer. Medullary carcinoma shows significant genomic instability with frequent chromosomal aberrations.
The pathogenesis of medullary carcinoma is not fully understood. It is widely believed to arise de novo rather than from identifiable precursor lesions other than possibly DCIS. Medullary carcinoma may have increased neoantigens, which are novel proteins presented on the cell surface derived from the accumulation of somatic mutations in tumor cells, which may boost the immune response against tumor cells. Neoantigens are associated with BRCA1 mutations and basal-like breast cancers, including medullary carcinoma.
Medullary carcinoma - radiologic and microscopic images
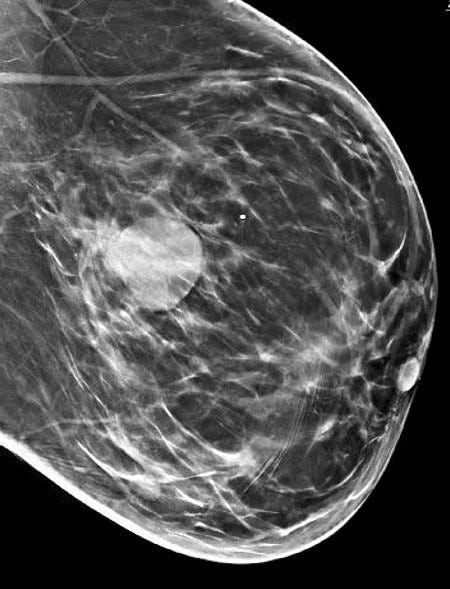
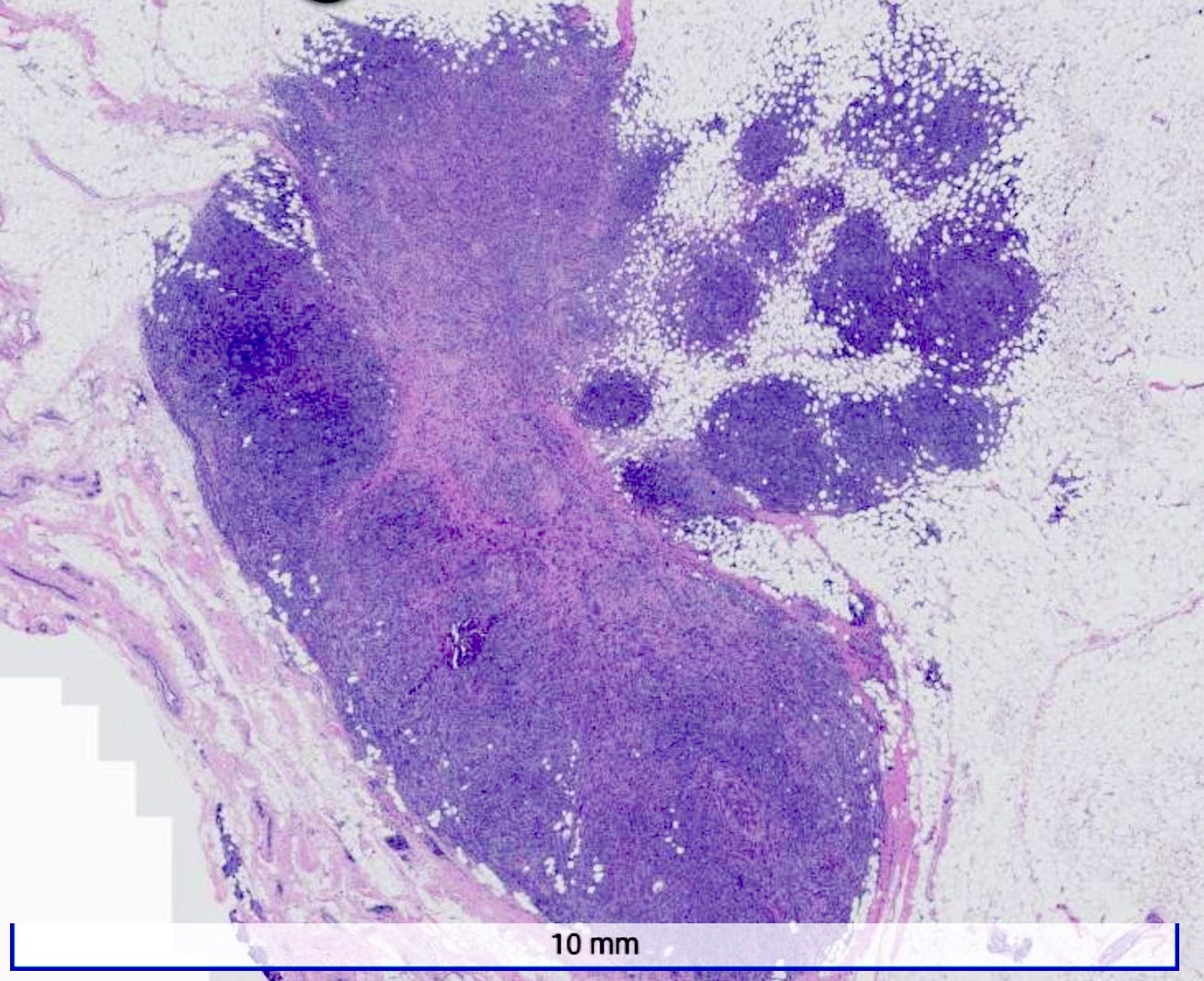
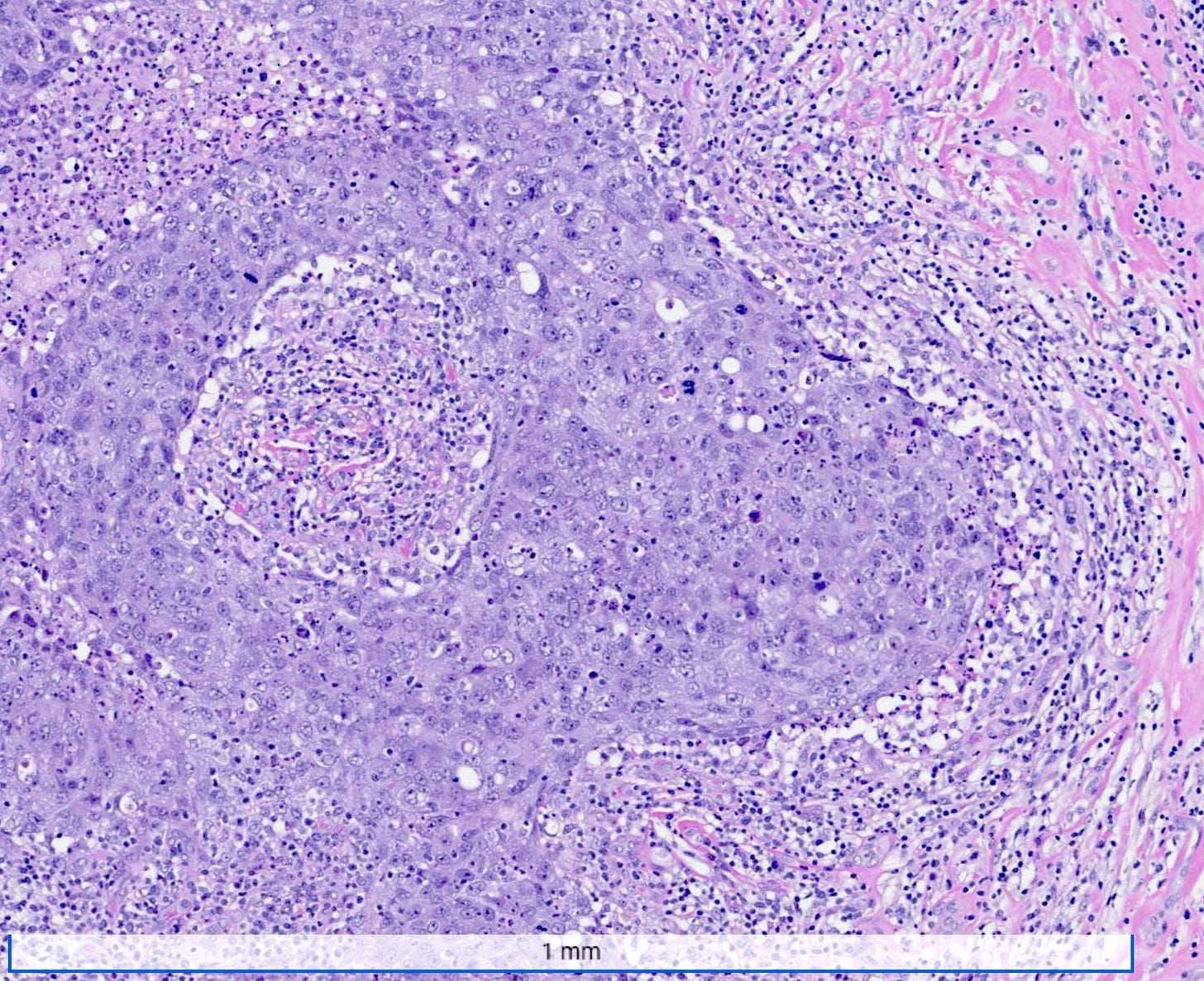
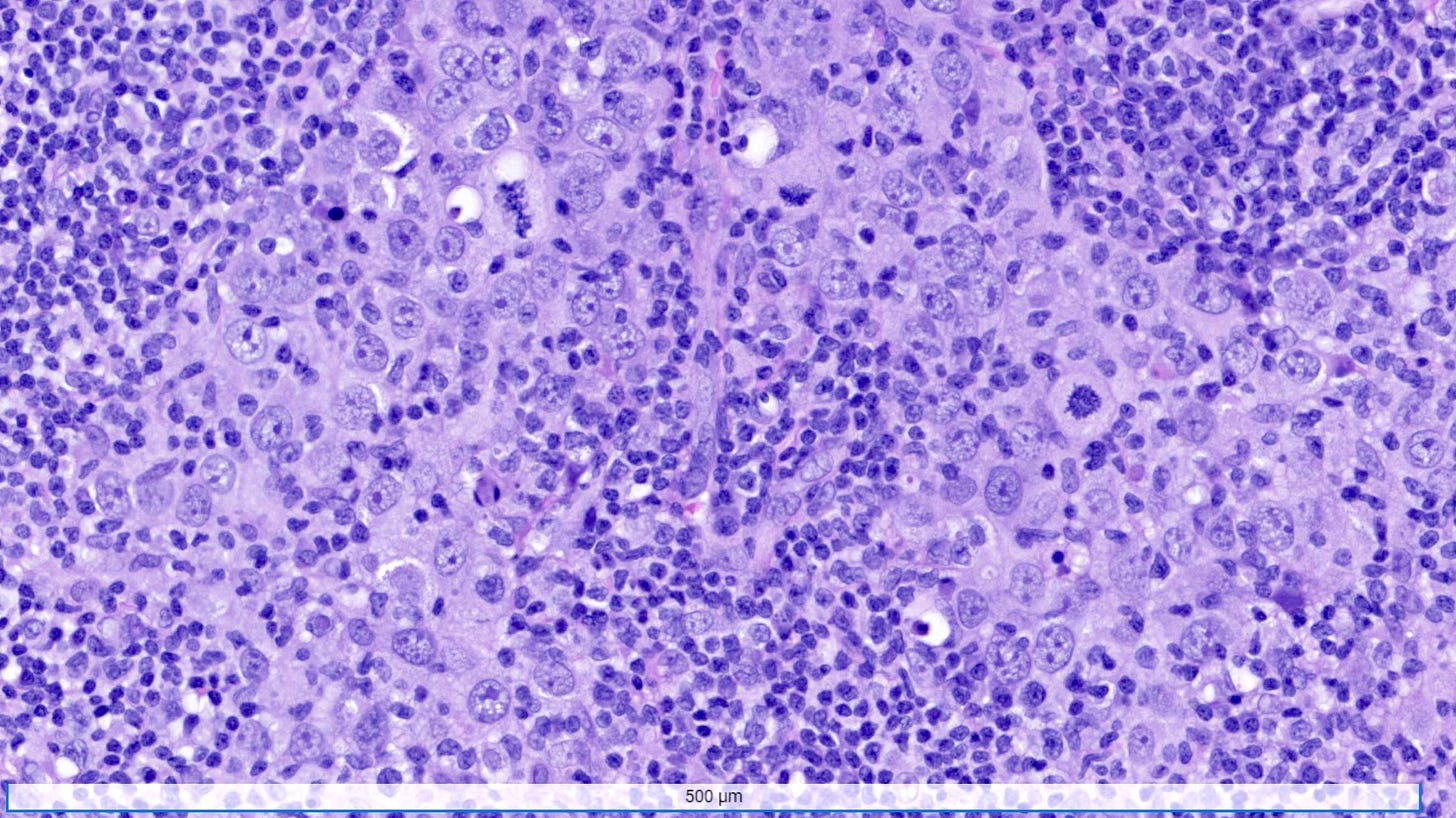
Adenoid cystic carcinoma of the breast
Adenoid cystic carcinoma of the breast is a rare subtype (< 0.1%) of invasive breast carcinoma. It resembles its salivary gland counterpart, characterized by a dual population of epithelial and myoepithelial cells.
Adenoid cystic carcinoma of the breast has a mean age at diagnosis of 63 years. Unlike other triple negative breast cancers, axillary lymph node metastases are rare (< 2% of cases). The 5 year disease free survival rate approaches 100% and recurrences are rare.
Surgical excision is the mainstay of treatment, with possible radiation therapy. Given the low incidence of lymph node involvement, axillary lymph node dissection is generally not recommended.
Grossly, adenoid cystic carcinoma of the breast typically presents as a 1 - 3 cm, well circumscribed, firm mass.
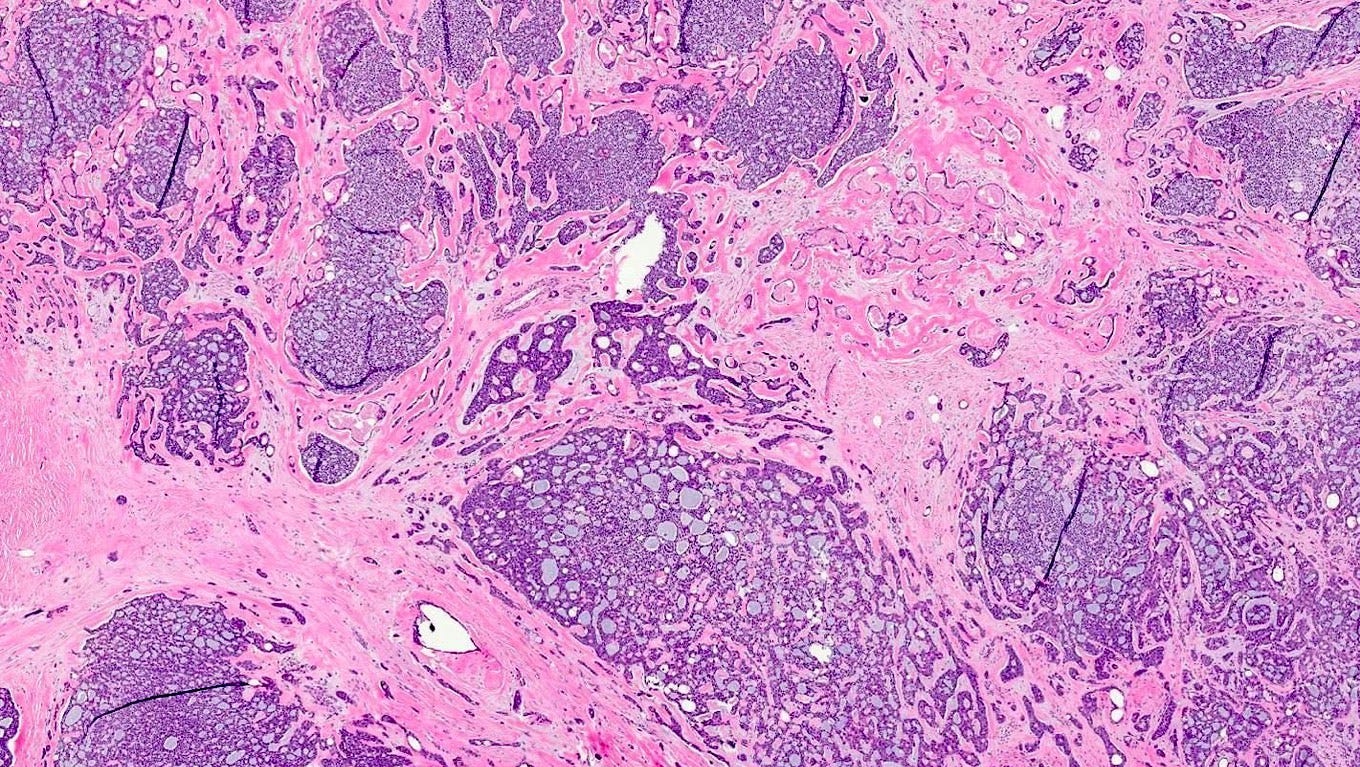
Microscopically, adenoid cystic carcinoma of the breast is characterized by a biphasic cellular pattern of luminal epithelial and basaloid myoepithelial cells. The tumor exhibits cribriform, tubular and solid growth patterns, with pseudocystic spaces filled with basophilic mucinous material. Perineural invasion is less common in the breast, distinguishing it from its salivary gland counterpart.
Fine needle aspiration cytology shows clusters of epithelial cells oriented around solid spheres of basement membrane material.
Immunohistochemically, adenoid cystic carcinoma of the breast is typically triple negative (i.e., negative for ER, PR and HER2).
Most cases are associated with the MYB::NFIB fusion gene due to translocation t(6;9)(q22-23;p23-24), which is also seen in salivary gland cases. This fusion gene may trigger cell proliferation, survival and cell cycle progression. Risk factors for this translocation are unknown. To our knowledge, salivary gland metaplasia only appears in the breast with benign (pleomorphic adenoma) or malignant tumors (acinic cell carcinoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, polymorphous adenocarcinoma), with no precursor lesions identified for the malignant processes. Since the t(6;9)(q22-23;p23-24) translocation only appears to be associated with salivary gland tumors, we speculate that the metaplasia appears first as part of a generalized malignant transformation. This is then followed by the translocation, which promotes the distinctive microscopic features of adenoid cystic carcinoma.
Adenoid cystic carcinoma of the breast should be distinguished from collagenous spherulosis and invasive cribriform carcinoma, which it resembles.
Adenoid cystic carcinoma of the breast - microscopic and cytologic images
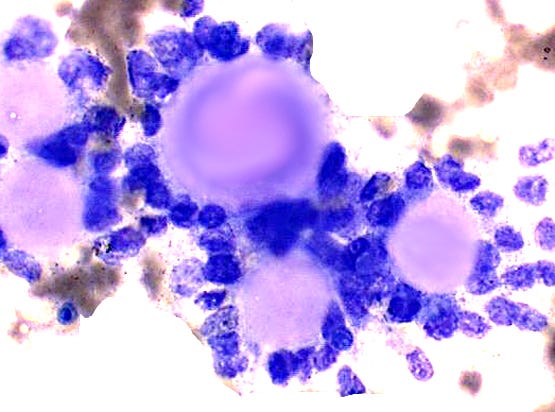
Polymorphous adenocarcinoma of the breast
Polymorphous adenocarcinoma of the breast is an exceptionally rare subtype of invasive breast carcinoma, with only 4 cases reported in the medical literature as of 2023. It shares morphological features with its salivary gland counterpart and is characterized by a diverse architectural pattern and uniform cytology.
Polymorphous adenocarcinoma of the breast has been observed in women aged 37 to 74 years. In reported cases, it presented as a palpable mass 1.5 to 4 cm in diameter.
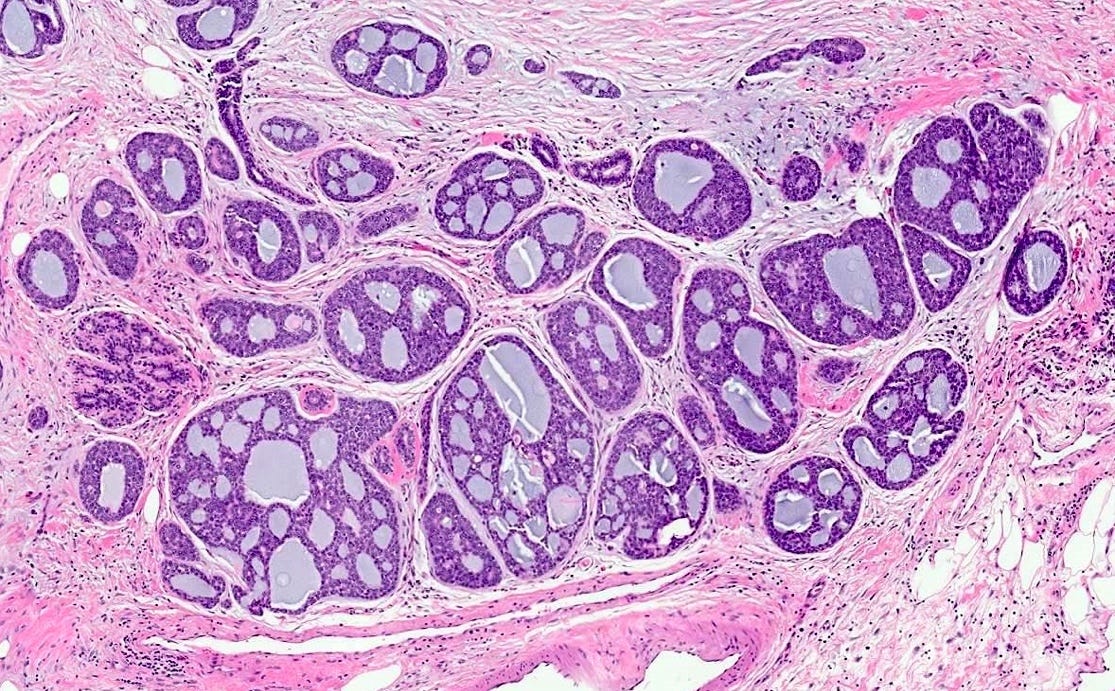
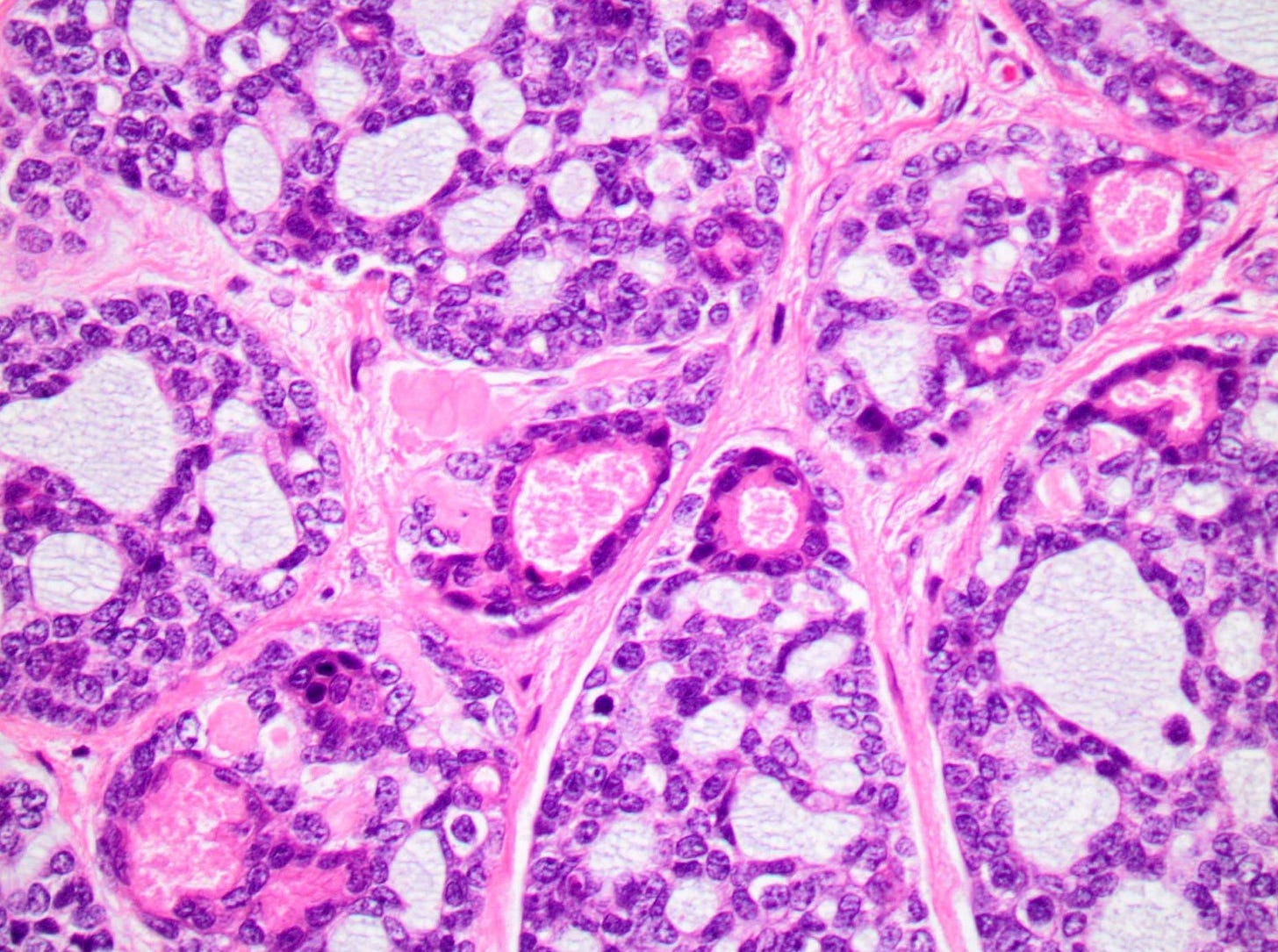
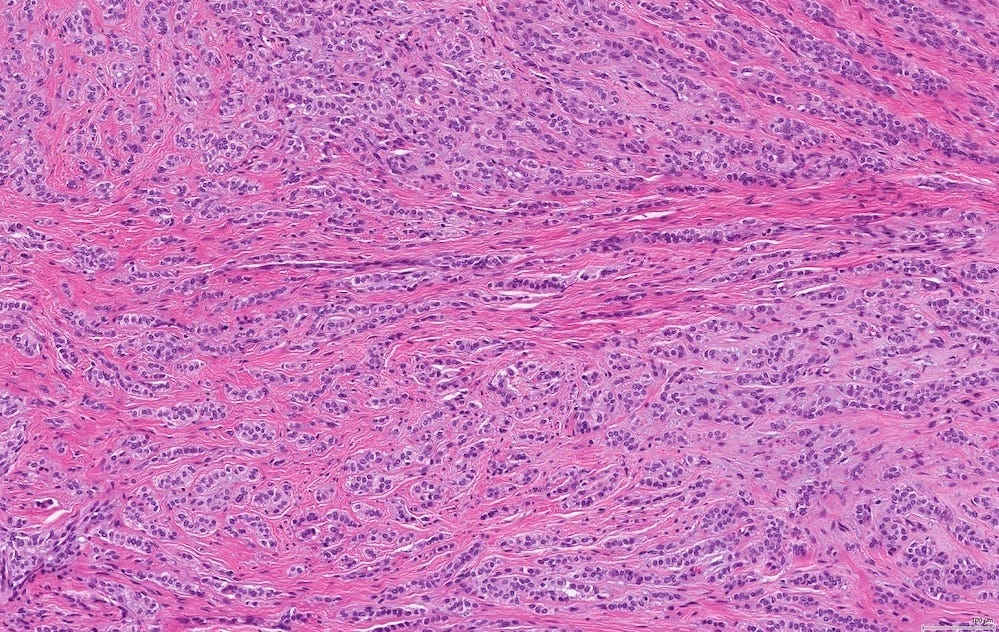
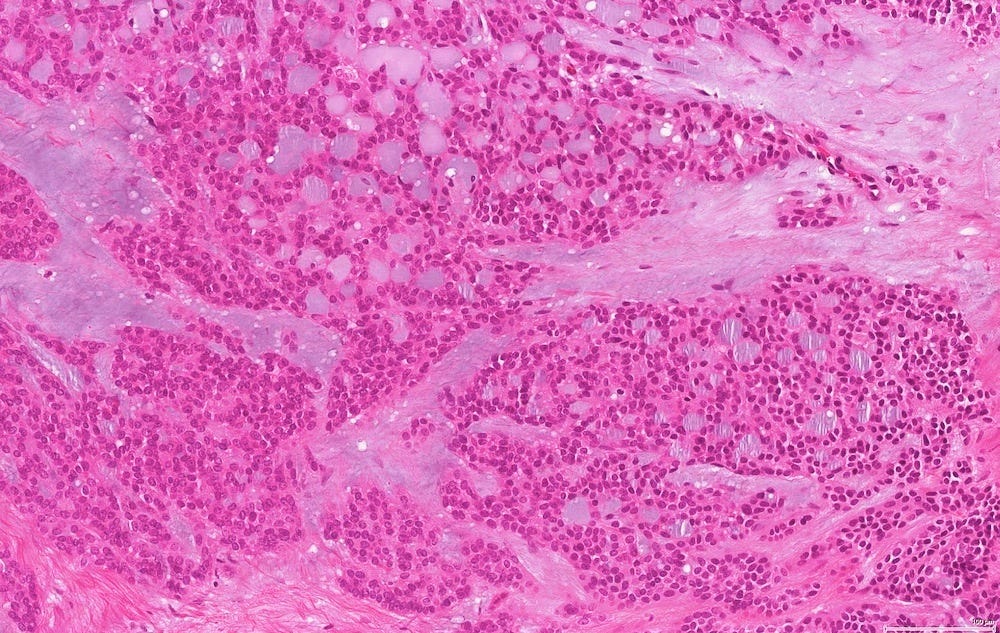
In reported cases, the tumor appears as a well circumscribed, firm mass. Microscopically, reported cases exhibited a wide spectrum of growth patterns, including solid nests, trabeculae, tubules, cribriform structures, strands and fascicles. In these cases, the tumor cells were monotonous with uniform nuclei and scant cytoplasm, lacking significant pleomorphism or mitotic activity.
Reported cases exhibited a triple negative phenotype (i.e., negative for ER, PR and HER2). They expressed basal-like markers such as cytokeratin 5/6 and p63. Salivary gland tumors typically express the PRKD1 E710D hotspot mutation, but the presence of this mutation in breast tumors is unknown.
Surgical excision with clear margins was the primary treatment modality in these cases. The role of adjuvant therapies, including chemotherapy, radiation and hormonal therapy, remains unclear due to the limited number of cases and lack of long term followup data.
Given the uncertainty regarding long term outcomes, close clinical follow-up may be prudent.
Due to its rarity, there are no established risk factors or precursor lesions identified for this tumor.
Differential diagnoses include other salivary gland-like tumors of the breast.
Polymorphic carcinoma - microscopic images
Note: due to its rarity in the breast, these images are from the similar tumor in the salivary glands
Tall cell carcinoma with reverse polarity of the breast
Tall cell carcinoma with reverse polarity is rare in the breast (< 100 cases reported) but resembles the tall cell variant of papillary thyroid carcinoma histologically, although it has different molecular characteristics.
It predominantly affects women with a mean age at diagnosis of 64 years and a relatively indolent course. Axillary lymph node involvement is uncommon and distant metastases are infrequent.
Surgical excision may be adequate therapy. Axillary nodal dissection, chemotherapy, radiation therapy and hormonal therapy may not be indicated.
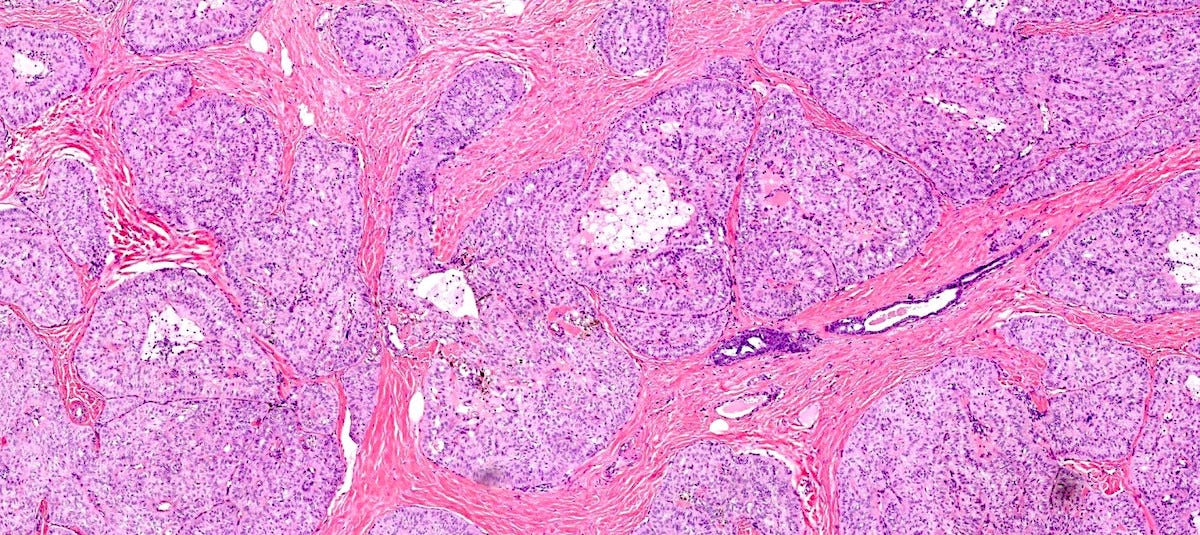
Tall cell carcinoma with reverse polarity presents as a 1 - 3 cm, well circumscribed, firm mass. Microscopically, it is characterized by elongated epithelial cells with abundant glassy eosinophilic cytoplasm and with nuclei located at the apical pole rather than the basal pole, giving the impression of reverse nuclear polarity. Architectural patterns include papillary, tubular and solid formations. In addition, it has papillary thyroid carcinoma features including colloid-like material within luminal spaces, nuclear grooves and overlapping nuclei.
Fine needle aspiration cytology may reveal cohesive clusters of tall epithelial cells with granular cytoplasm and nuclear atypia, although there are limited reports.
Tall cell carcinoma with reverse polarity is typically triple negative (i.e., negative for ER, PR and HER2). It is often positive for breast epithelial markers GATA3, GCDFP-15 and mammaglobin but is negative for thyroid specific markers TTF1 and thyroglobulin.
The differential diagnosis includes metastatic tall cell variant of papillary thyroid carcinoma (use immunohistochemistry and molecular testing to differentiate) and apocrine carcinoma (different nuclear features and immunohistochemistry).
Tall cell carcinoma with reverse polarity of the breast arises from an IDH2 R172 hotspot mutation (present in 77 - 100% of cases) and a PIK3CA hotspot mutation (present in 67% of cases), which are not found in the thyroid tumor. To date, no precursor lesion has been identified for tall cell carcinoma with reverse polarity in the breast. How the IDH2 mutation arises is unknown, but it is also present in gliomas, leukemias and other malignancies.
Tall cell carcinoma with reverse polarity - microscopic images
The next essay, part 6m will discuss other triple negative breast cancers without known precursors.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me on LinkedIn, Threads and Instagram (@npernickmich) or Bluesky (@natpernick.bsky.social).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week, we post interesting cancer related images of malignancies with diagnoses, plus articles of interest. Please also read our CCN essays.
Latest cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email: Nat@PathologyOutlines.com (please note I cannot provide medical advice)
Follow my Substack Notes — subscribers are automatically notified.


Thank you for the information. I'm still waiting for my friends diagnosis of which type of breast cancer his wife has. I know there is no lymph node indications at this time.