Cancer precursor project - breast cancer, part 6n
22 June 2025
This weekly medical blog focuses on science, not politics. For political commentary, please refer to my separate daily blog. Feel free to skip any essays or sections that are not of interest — no offense taken.
This post is part of my cancer precursor project, which explores how cancer arises. Part 6 addresses breast cancer. Click here for links to essays on all 46 types of breast cancer (some are still in progress).
In this essay, I discuss neuroendocrine types of breast cancer without known precursors: neuroendocrine tumor, grade 1, neuroendocrine tumor, grade 2 and neuroendocrine carcinoma (small cell and large cell). I have previously written about small cell carcinoma, which may arise from ductal carcinoma in situ (DCIS).
Neuroendocrine breast cancers should be distinguished from other breast tumors that exhibit neuroendocrine differentiation, such as solid papillary carcinoma (which is typically intraductal with mucin production), mucinous carcinoma (characterized by tumor cells floating in extracellular mucin) and metastatic neuroendocrine tumors from other primary sites (most commonly lung or gastrointestinal tract , and can be distinguished by clinical history, lack of an in situ component [DCIS] and imaging findings).
Neuroendocrine neoplasms of the breast are rare. They are categorized into well differentiated neuroendocrine tumors (grades 1 and 2), neuroendocrine carcinoma and invasive breast cancer of no special type with neuroendocrine differentiation in less than 90% of tumor cells.
The origin of these tumors is uncertain. The most widely accepted theory is divergent neuroendocrine differentiation of neoplastic stem cells. This theory is supported by the clonal relationship of these tumors to breast carcinomas. An alternative theory, transformation from native neuroendocrine cells in the breast, is controversial, as these cells have not been clearly identified in normal breast tissue.
Neuroendocrine tumor of the breast, Grades 1 and 2
Neuroendocrine tumor, grade 1, previously termed carcinoid tumor, is a primary (i.e. not metastatic), low grade breast neoplasm. It has neuroendocrine morphology in at least 90% of cells and is immunoreactive to neuroendocrine markers. It is well differentiated, has a low mitotic index (i.e. Ki67 index < 3%) and no necrosis.
Neuroendocrine tumor, grade 2, previously known as atypical carcinoid tumor, is classified as intermediate grade (i.e. more aggressive than grade 1 but less aggressive than neuroendocrine carcinoma). It shows greater nuclear pleomorphism and higher mitotic activity than grade 1 tumors.
These grade 1 and 2 tumors predominantly affect women aged 60–70 years. There is no known hereditary association or specific risk factor that distinguishes them from other types of breast cancer. Clinical and imaging features are nonspecific.
Patients typically present with a palpable mass. Bilaterality and metastatic presentation are rare.
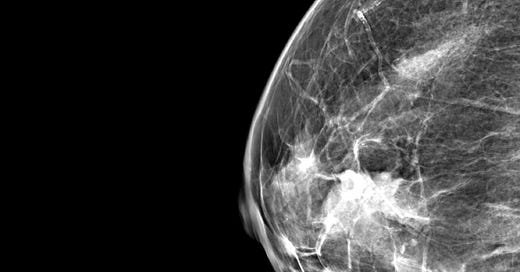
Mammography typically reveals a lobular, well defined dense mass without microcalcifications.
Treatment generally follows standard protocols for invasive breast carcinoma. This includes surgery, radiation and hormonal therapy if estrogen receptor (ER) or progesterone receptor (PR) expression is present. Chemotherapy is not routinely used.
Grade 1 neuroendocrine tumors are associated with favorable outcomes, similar to other low grade, hormone receptor positive breast cancers.
Grade 2 neuroendocrine tumors are rare, and long term outcome data are limited. They are considered to have an intermediate prognosis between grade 1 neuroendocrine tumors and neuroendocrine carcinoma.
Grossly, tumors may appear well circumscribed or infiltrative, with a firm, tan-white cut surface.
Microscopically, at least 90% of tumor cells must show neuroendocrine features:
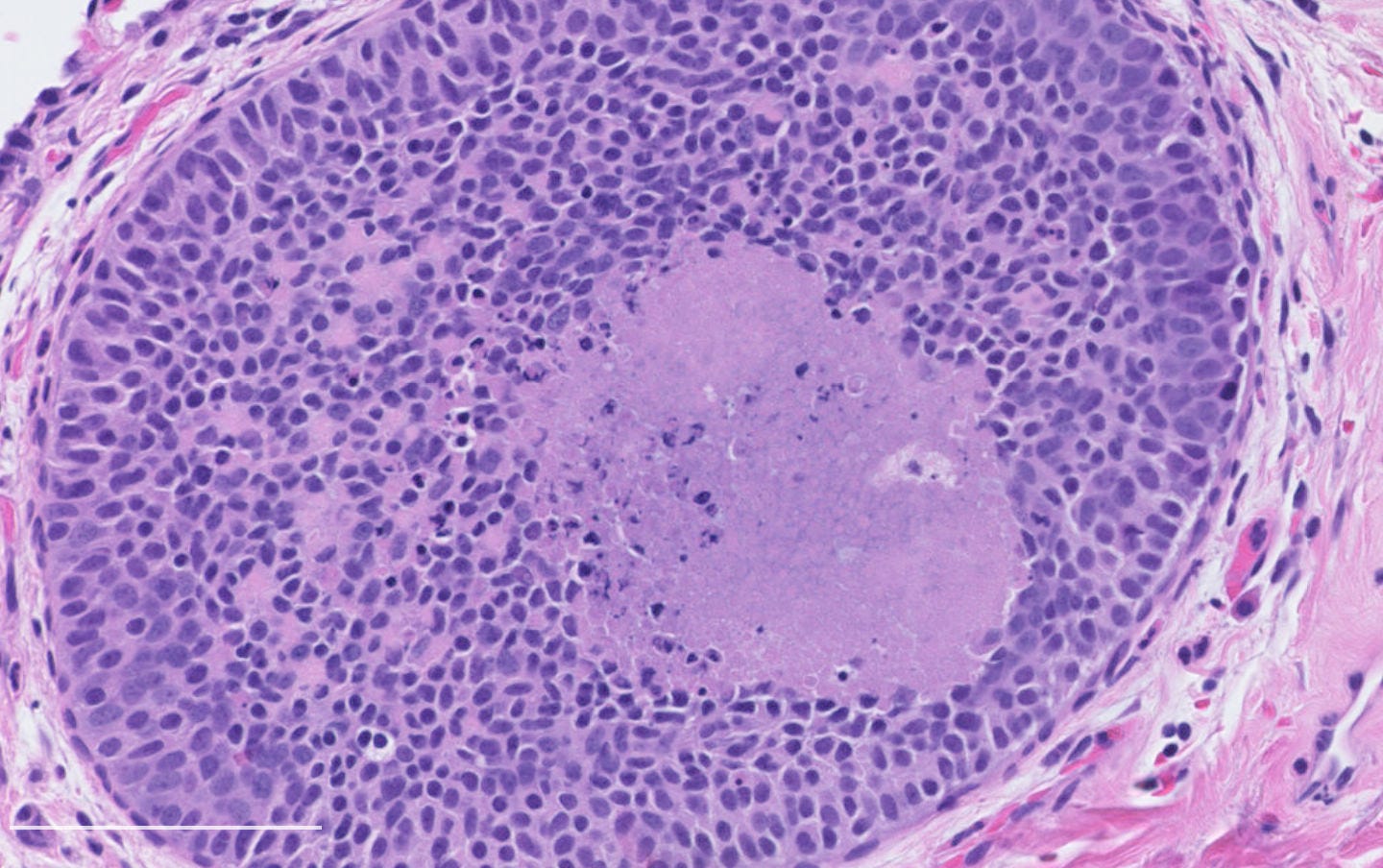
Architecture: Nested, trabecular or rosette-like patterns with delicate fibrous stroma.
Grade 1 tumors: Uniform small to medium sized cells with eosinophilic, granular cytoplasm and round to oval nuclei. Salt and pepper chromatin may be present but is not always prominent. Mitoses are rare (< 2 per 10 high-power fields), and necrosis is absent.
Grade 2 tumors: Show increased pleomorphism and mitotic activity and may show necrosis.
Immunohistochemical staining is positive for neuroendocrine markers including synaptophysin, chromogranin, INSM1, neuron specific enolase (NSE) and CD56 as well as hormonal markers ER and PR. HER2 is typically negative.
No specific genetic alterations are characteristic. However, some tumors may harbor mutations such as PIK3CA, which are also found in ER positive invasive ductal carcinomas.
Neuroendocrine tumor of the breast, grades 1 and 2 - radiologic, gross and microscopic images
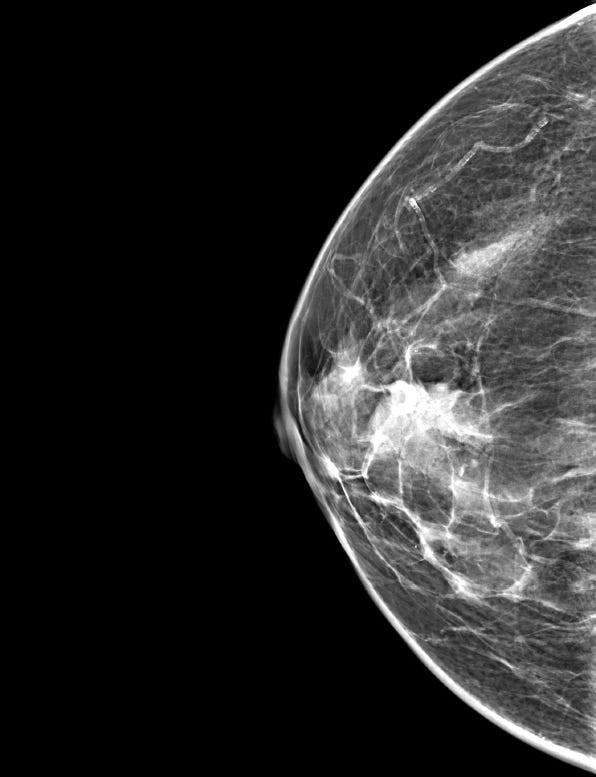
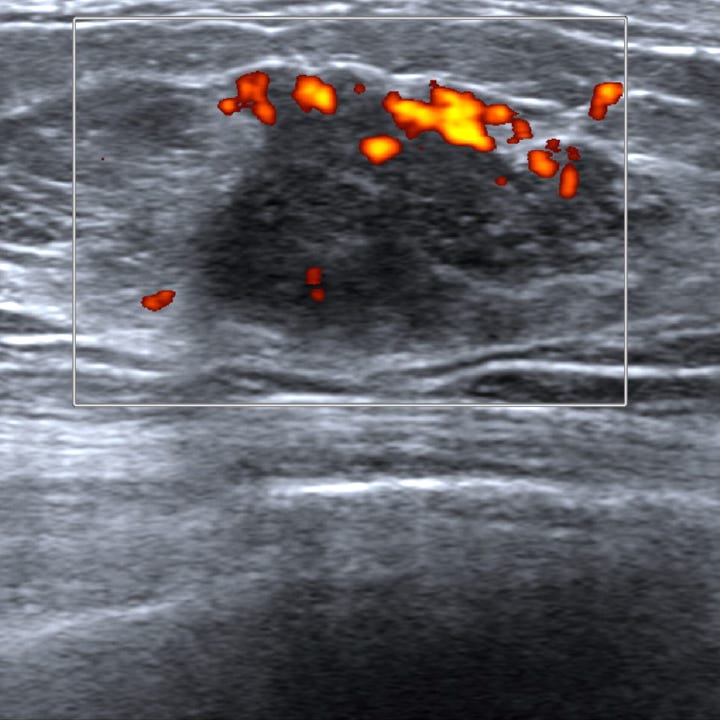
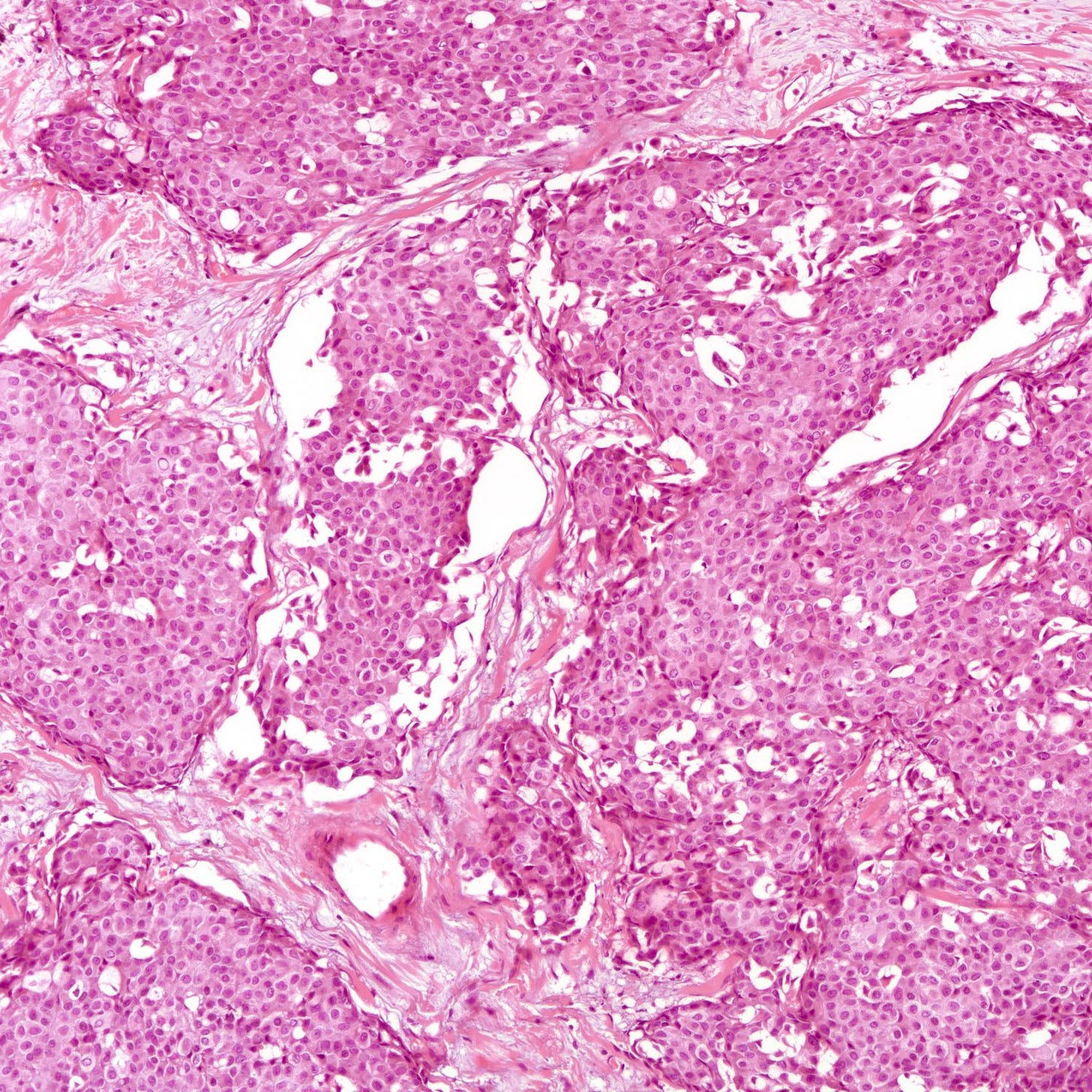
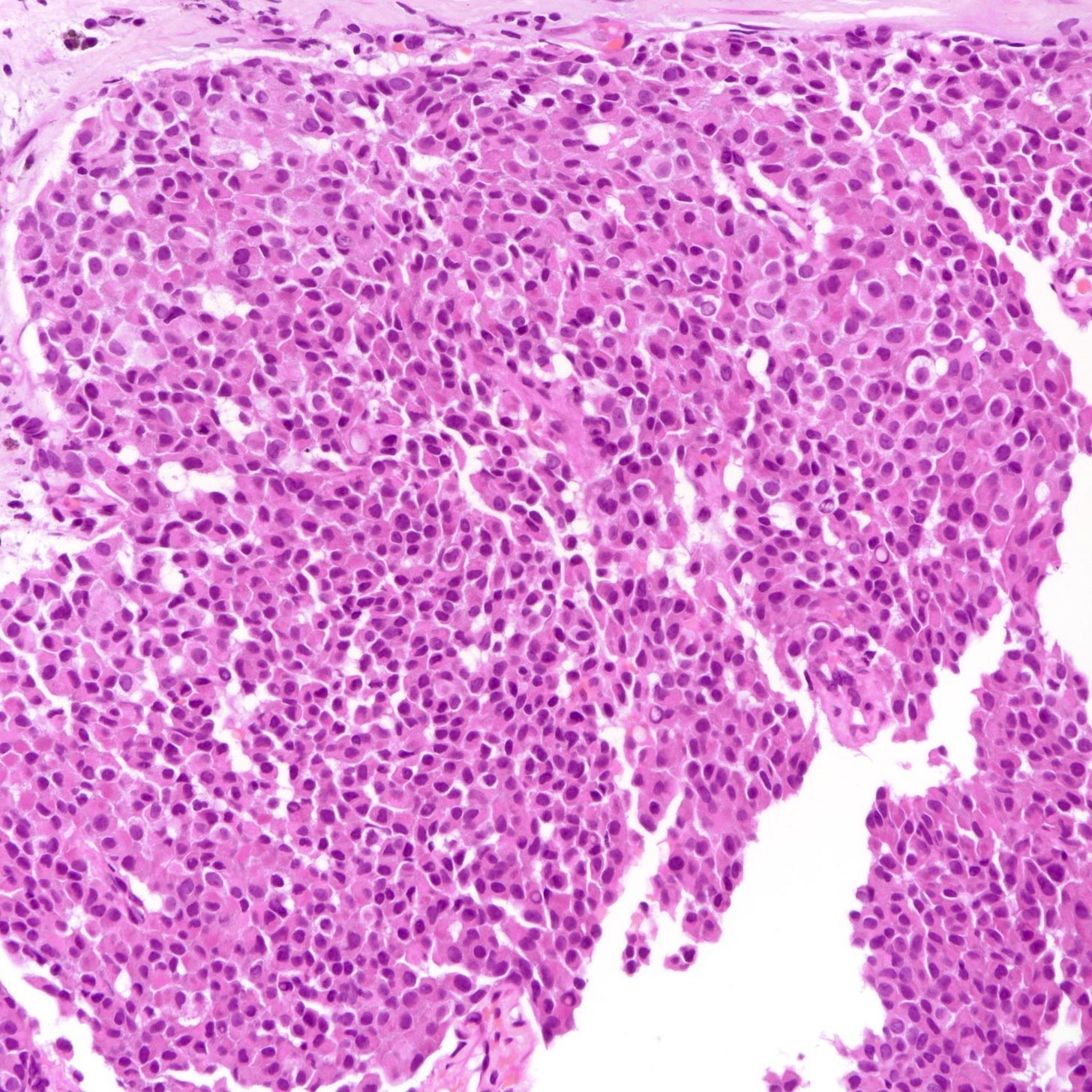

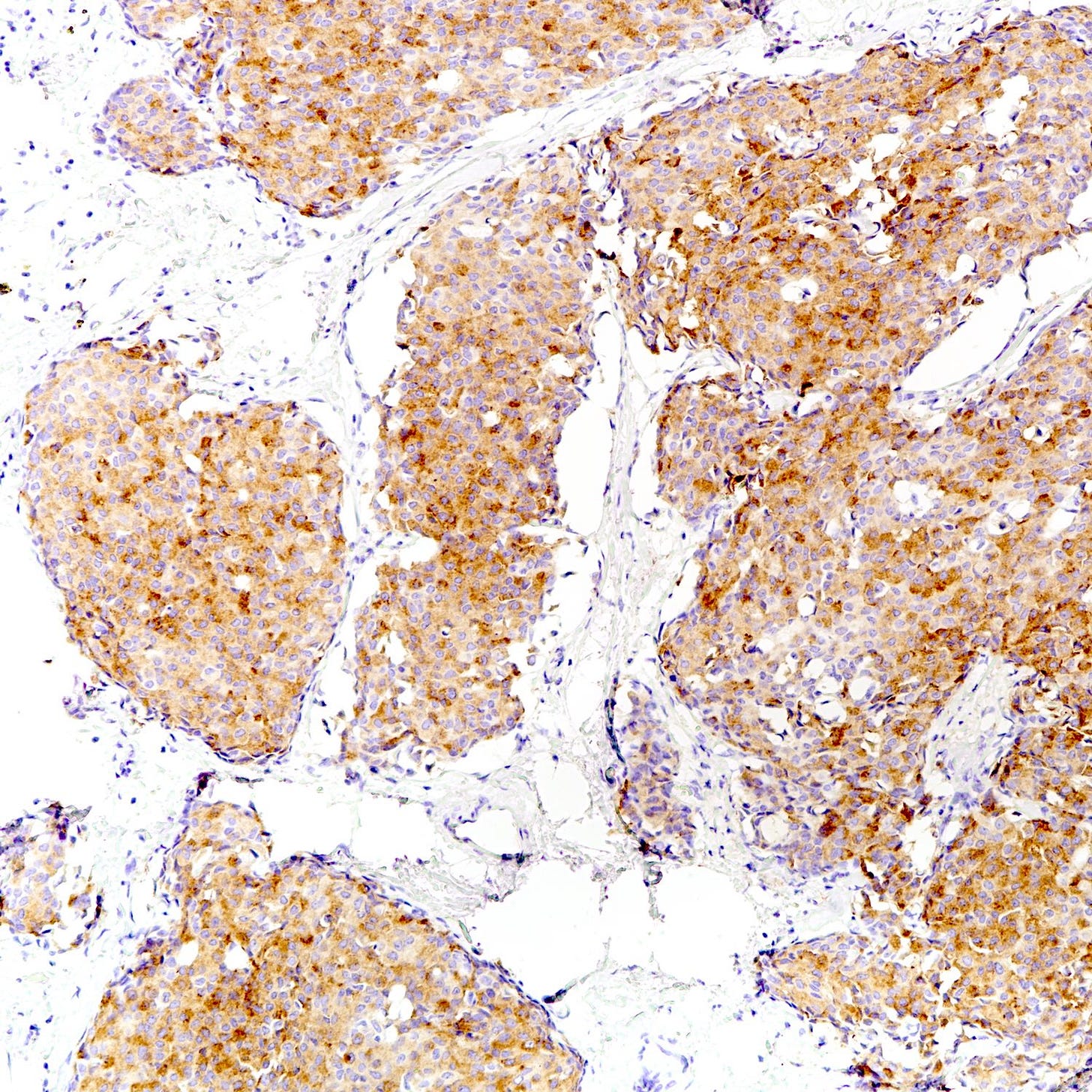
The following images show neuroendocrine tumor grade 2 from the lung, which is much more common than in the breast (for which I lack photos):
Neuroendocrine carcinoma of the breast
Neuroendocrine carcinoma of the breast is a rare, high grade malignant epithelial tumor characterized by neuroendocrine differentiation in at least 90% of tumor cells and histologic features of a poorly differentiated carcinoma. It is distinct from well differentiated neuroendocrine tumors (grades 1 and 2) and invasive breast carcinoma of no special type with focal neuroendocrine features (involves less than 90% of tumor cells). Neuroendocrine carcinoma includes both small cell and large cell subtypes, which resemble their pulmonary counterparts.
It is important to distinguish neuroendocrine carcinomas from other breast neoplasms with neuroendocrine differentiation, such as solid papillary carcinoma and mucinous carcinoma. These tumors are usually lower grade and have different clinical behavior.
A diagnosis of primary neuroendocrine carcinoma of the breast requires exclusion of another primary site (based on clinical history and imaging) and either the presence of ductal carcinoma in situ or an association with conventional invasive breast carcinoma.
Neuroendocrine carcinoma occurs predominantly in women over 60 years of age. There is no known genetic syndrome or specific risk factor associated with this tumor. It does not have distinctive clinical or radiologic features compared to other types of breast carcinoma.
Patients typically present with a unilateral palpable mass. Some may have nodal or distant metastases at diagnosis, reflecting the tumor’s aggressive nature.
On mammography, the tumor typically appears as a high density mass, often lacking microcalcifications. Ultrasound may reveal an irregular, hypoechoic lesion with increased vascularity.
Treatment follows the approach used for high grade invasive breast carcinoma, including surgery and chemotherapy. Endocrine therapy may be considered if hormone receptors are expressed. Due to its rarity, there is no standardized treatment regimen.
Prognosis is generally poor compared to conventional invasive breast carcinomas, with a high rate of recurrence and metastasis.
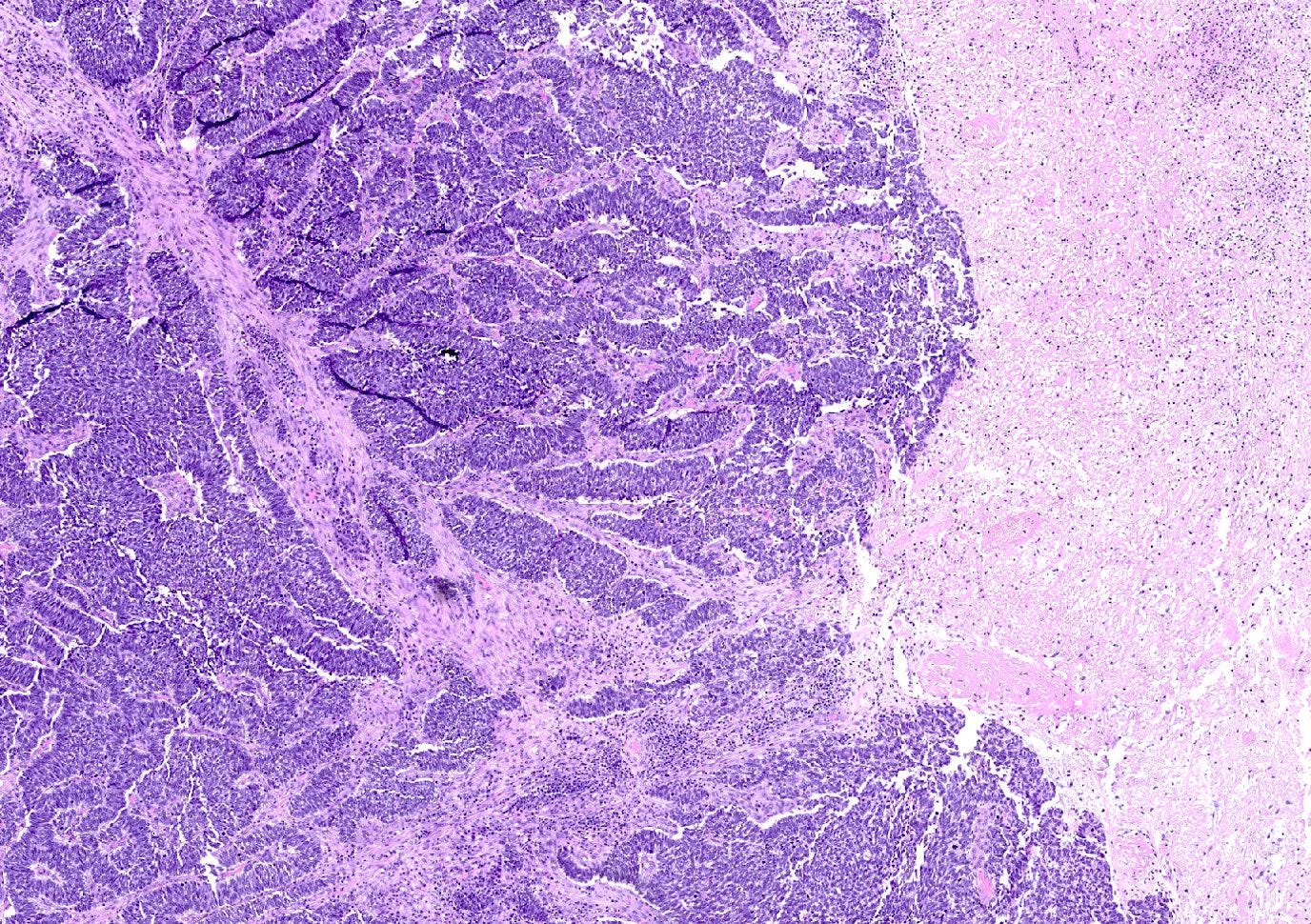
Grossly, neuroendocrine carcinoma may appear as a firm, ill defined mass with a tan-white cut surface. Necrosis and hemorrhage are often present.
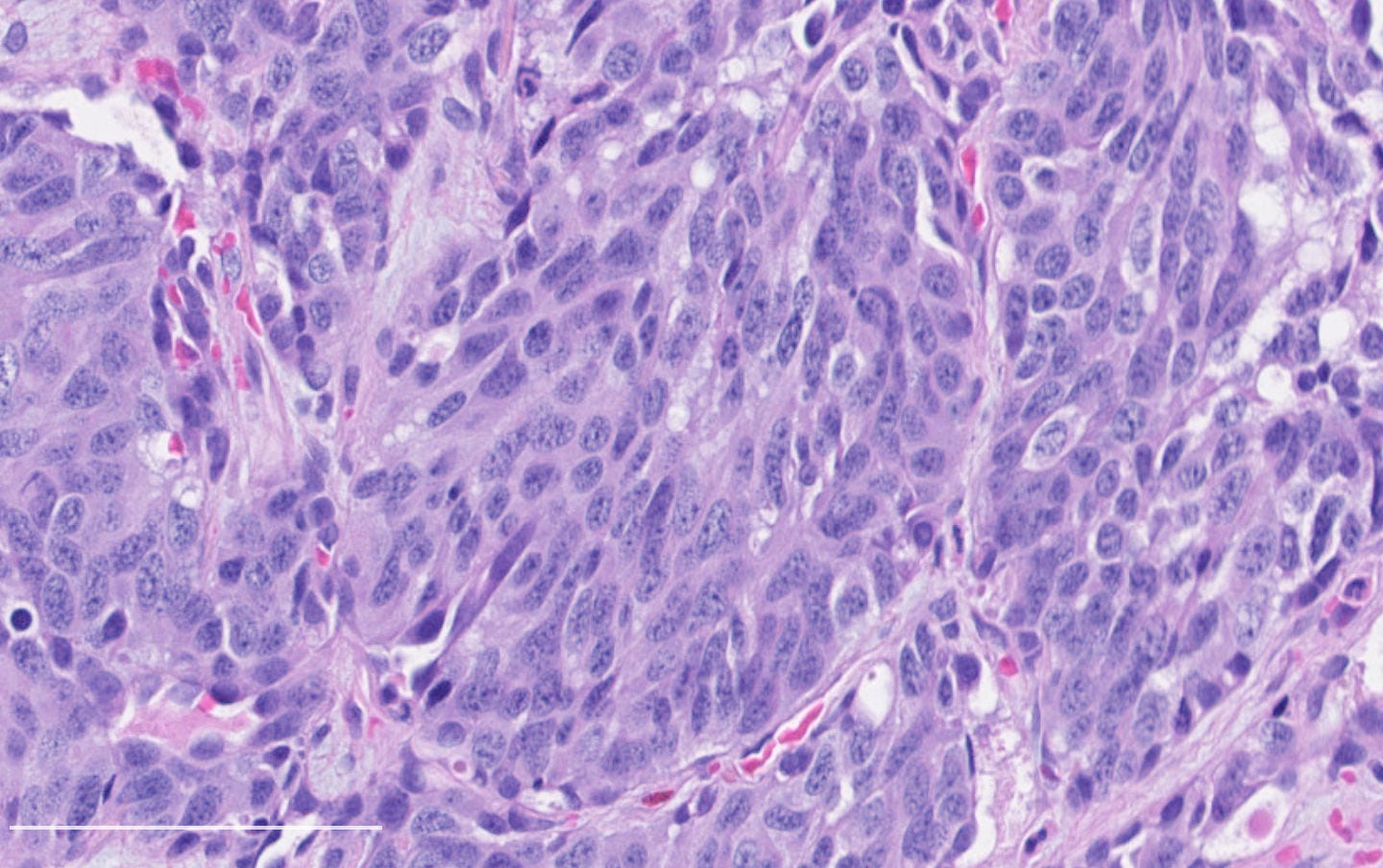
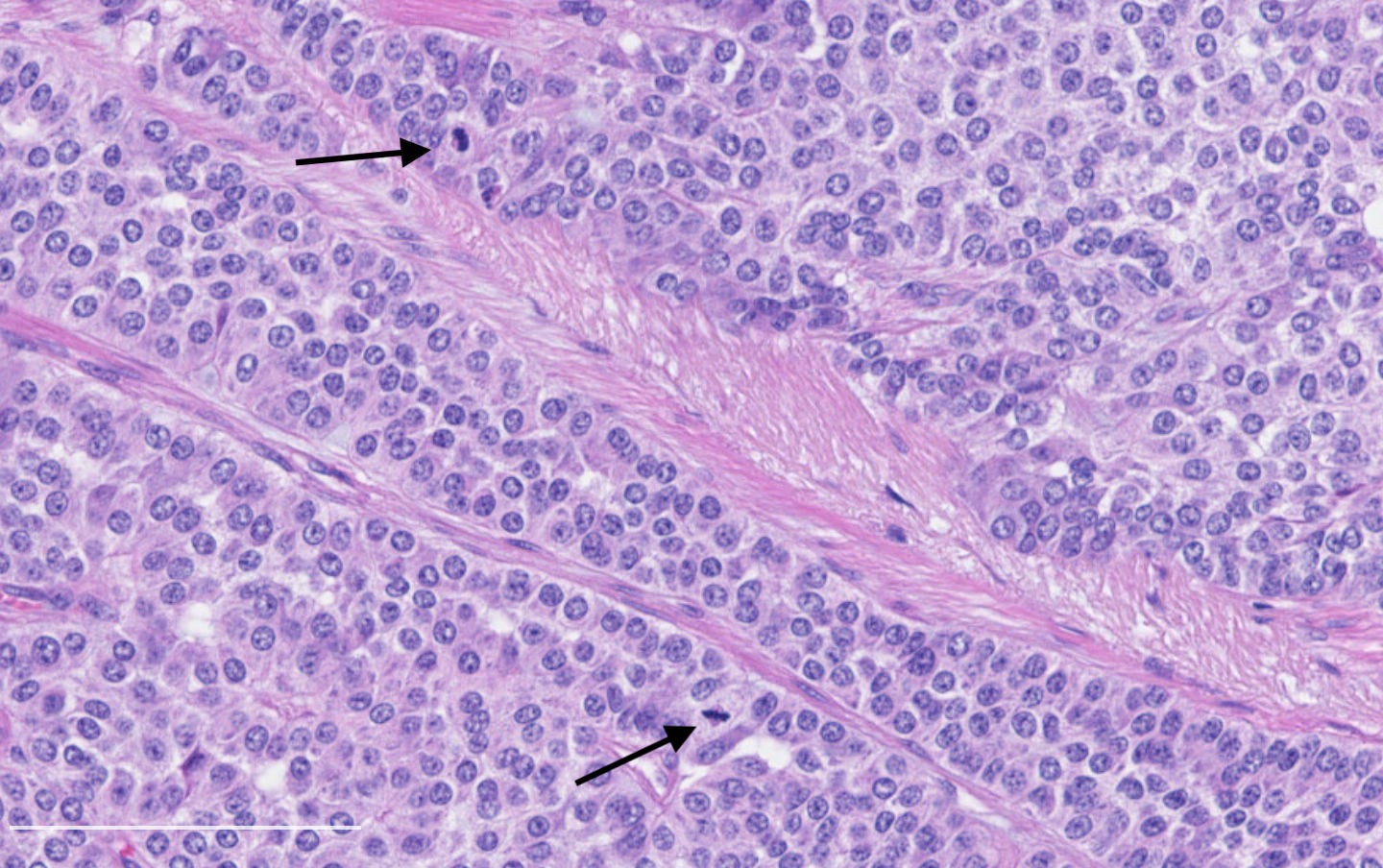
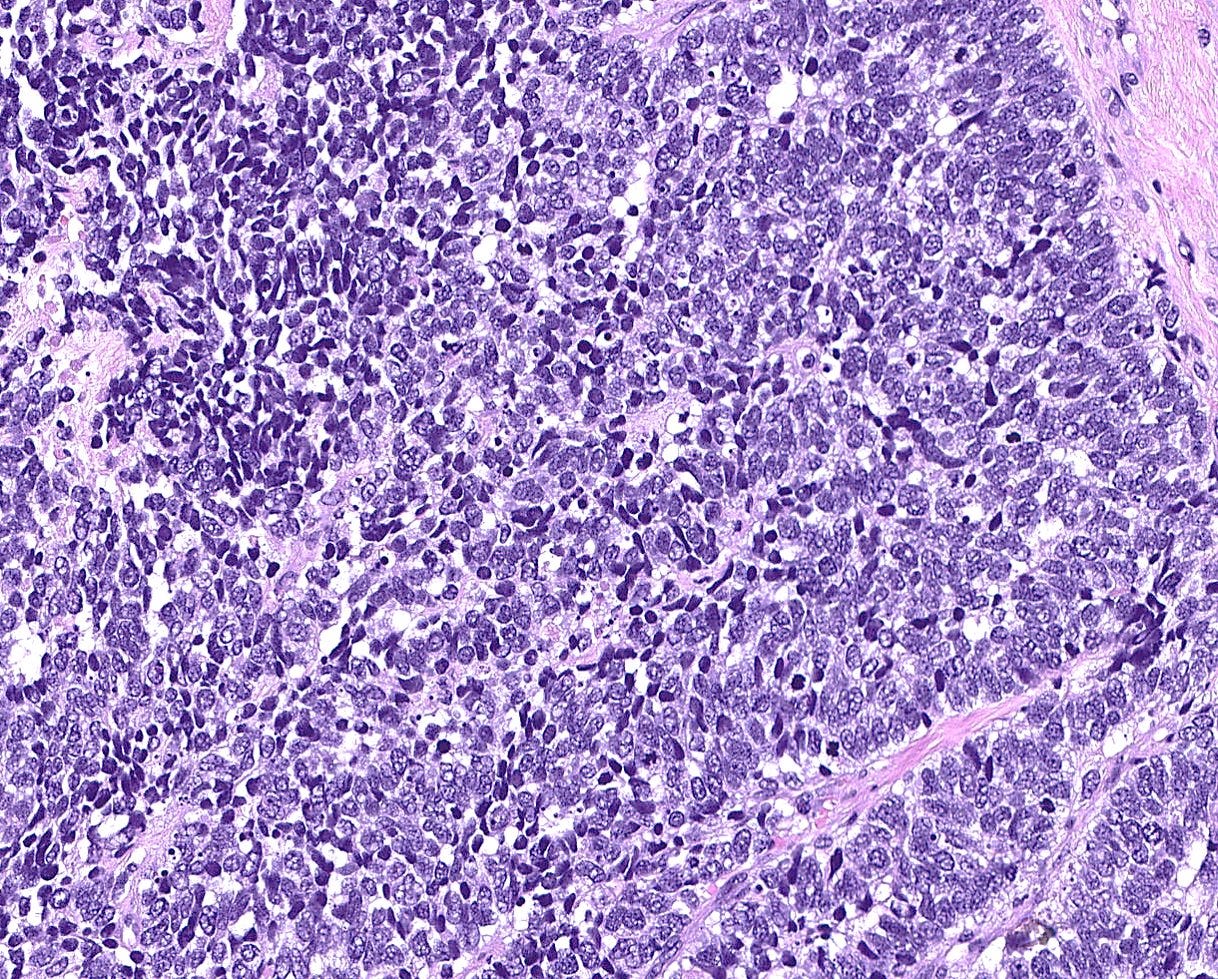
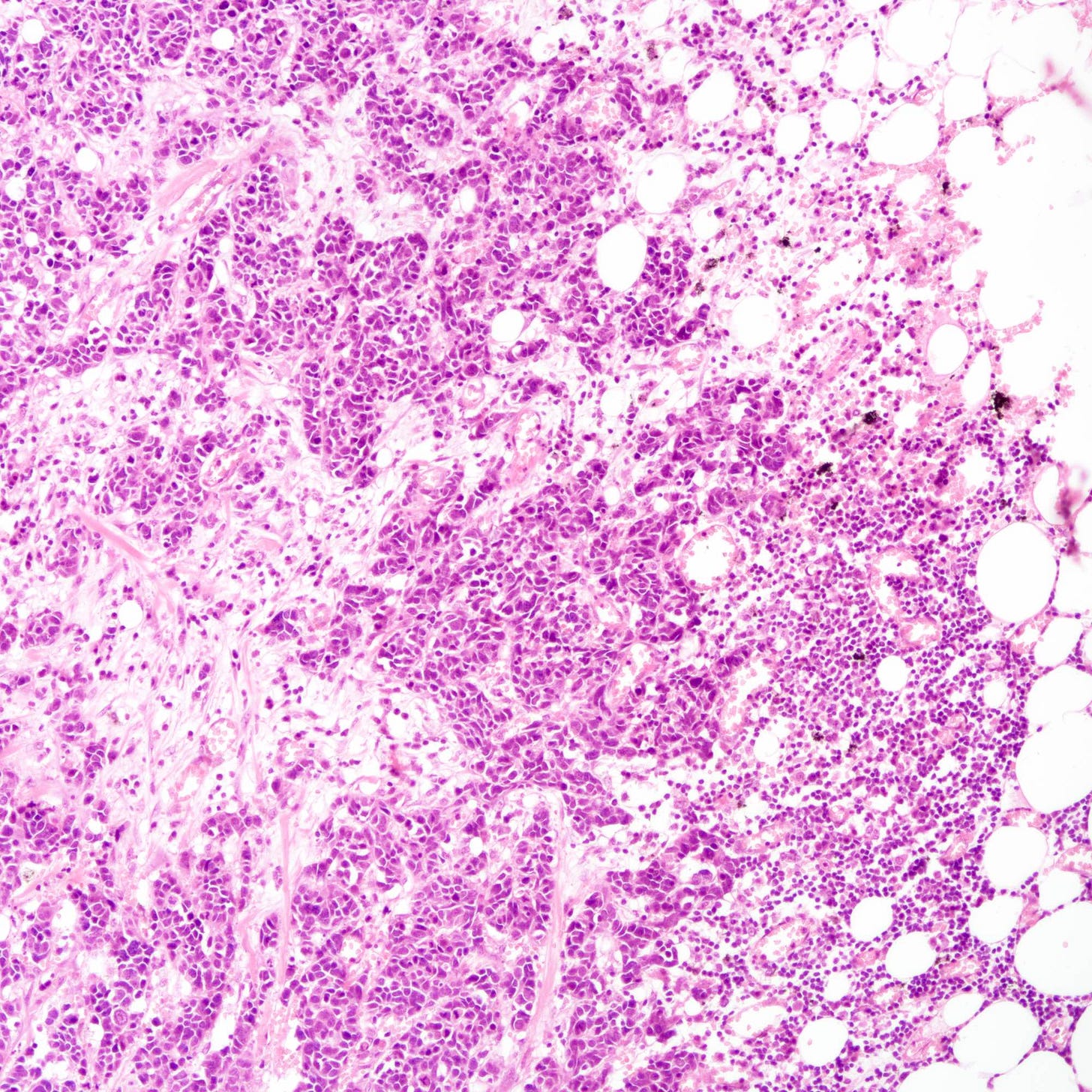
Histologically, these tumors are high grade and poorly differentiated and are either small cell or large cell type. Small cell carcinomas, described previously, consist of small cells with scant cytoplasm, high nuclear-to-cytoplasmic ratio, finely granular chromatin, inconspicuous nucleoli and nuclear molding. Crush artifact, brisk mitotic activity, lymphovascular invasion and necrosis are frequent. These tumors are often associated with proliferative changes, in situ carcinoma or conventional invasive carcinoma elsewhere in the breast.
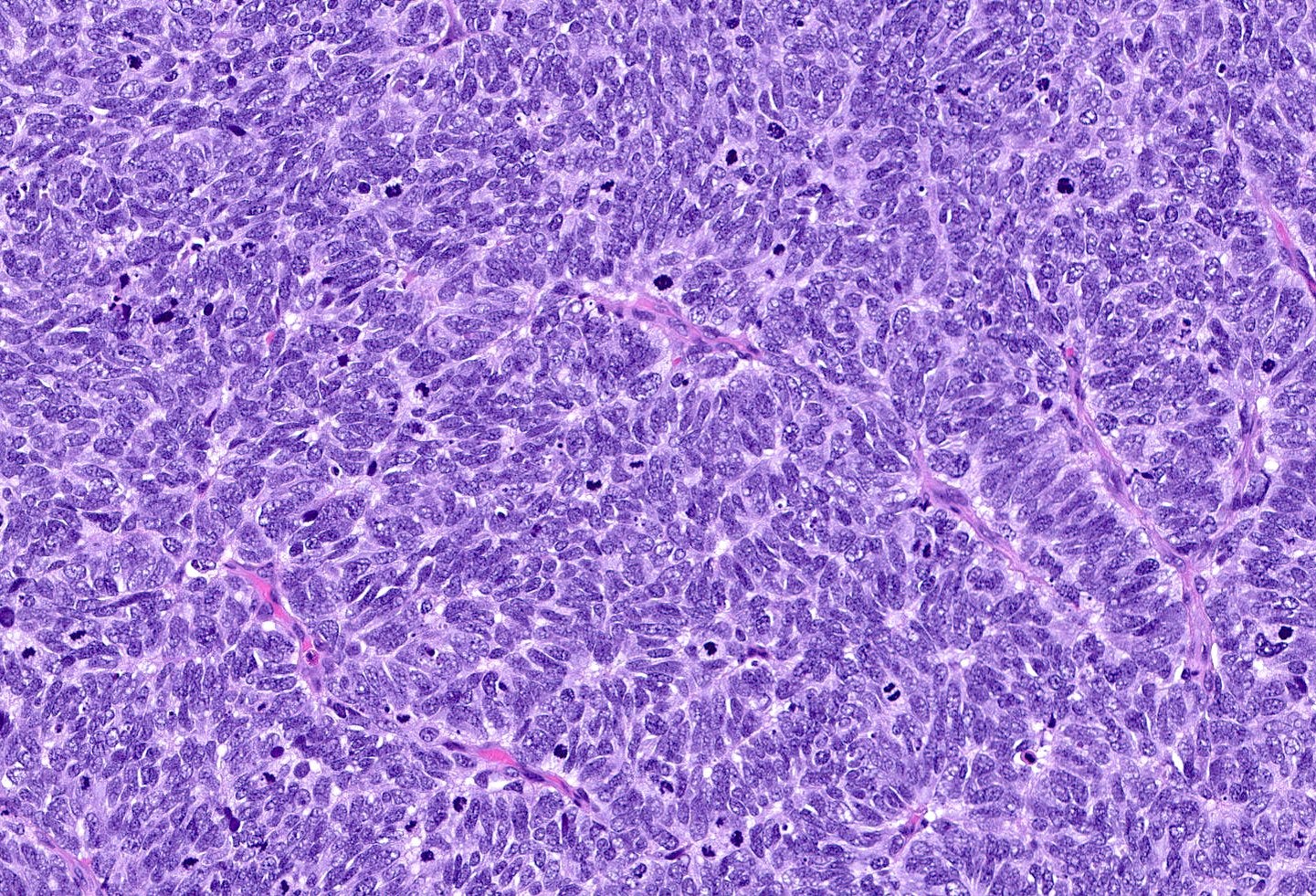
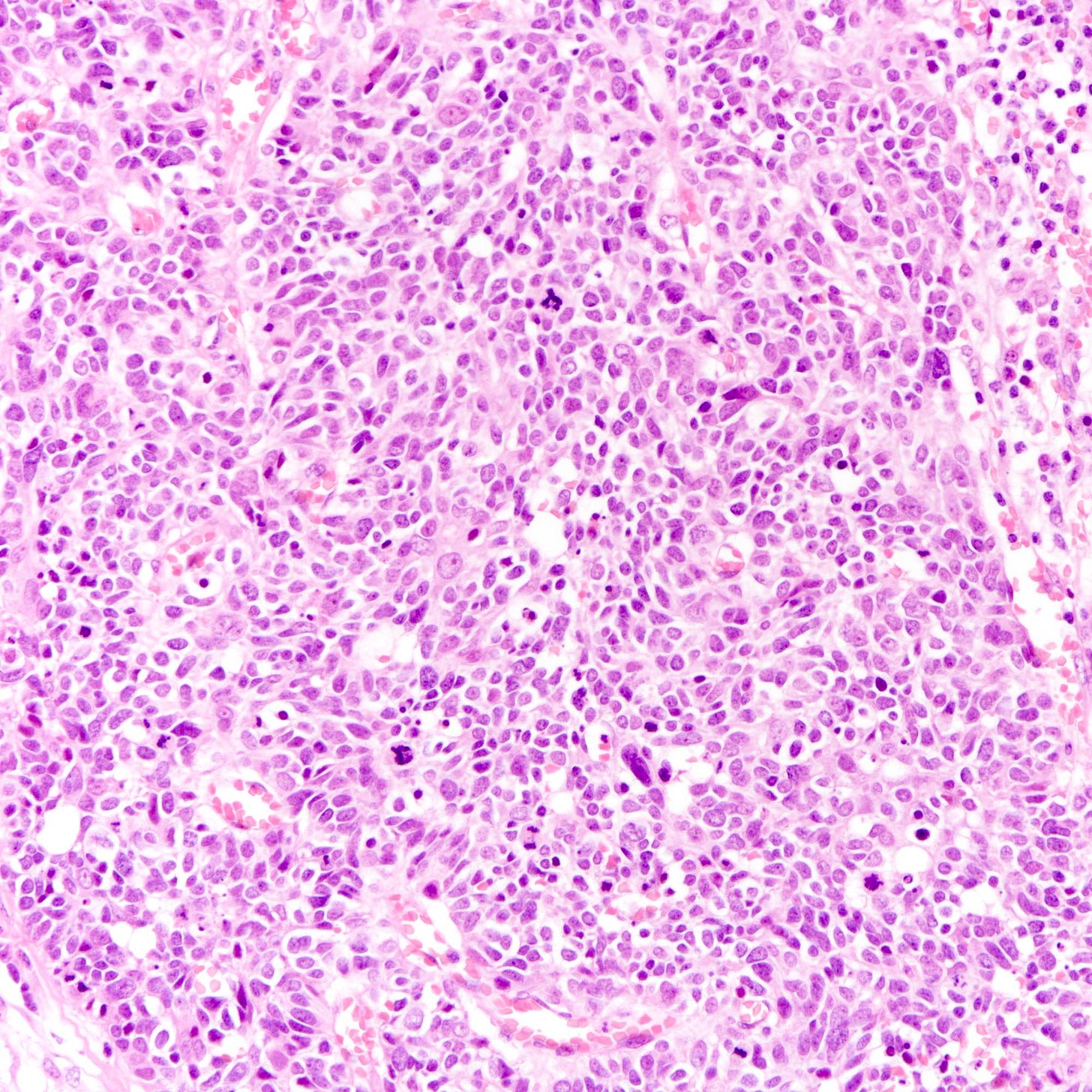
Large cell neuroendocrine carcinomas are composed of larger cells with more abundant cytoplasm, vesicular nuclei and occasional prominent nucleoli. Like small cell tumors, they exhibit high mitotic activity, lymphovascular invasion and necrosis.
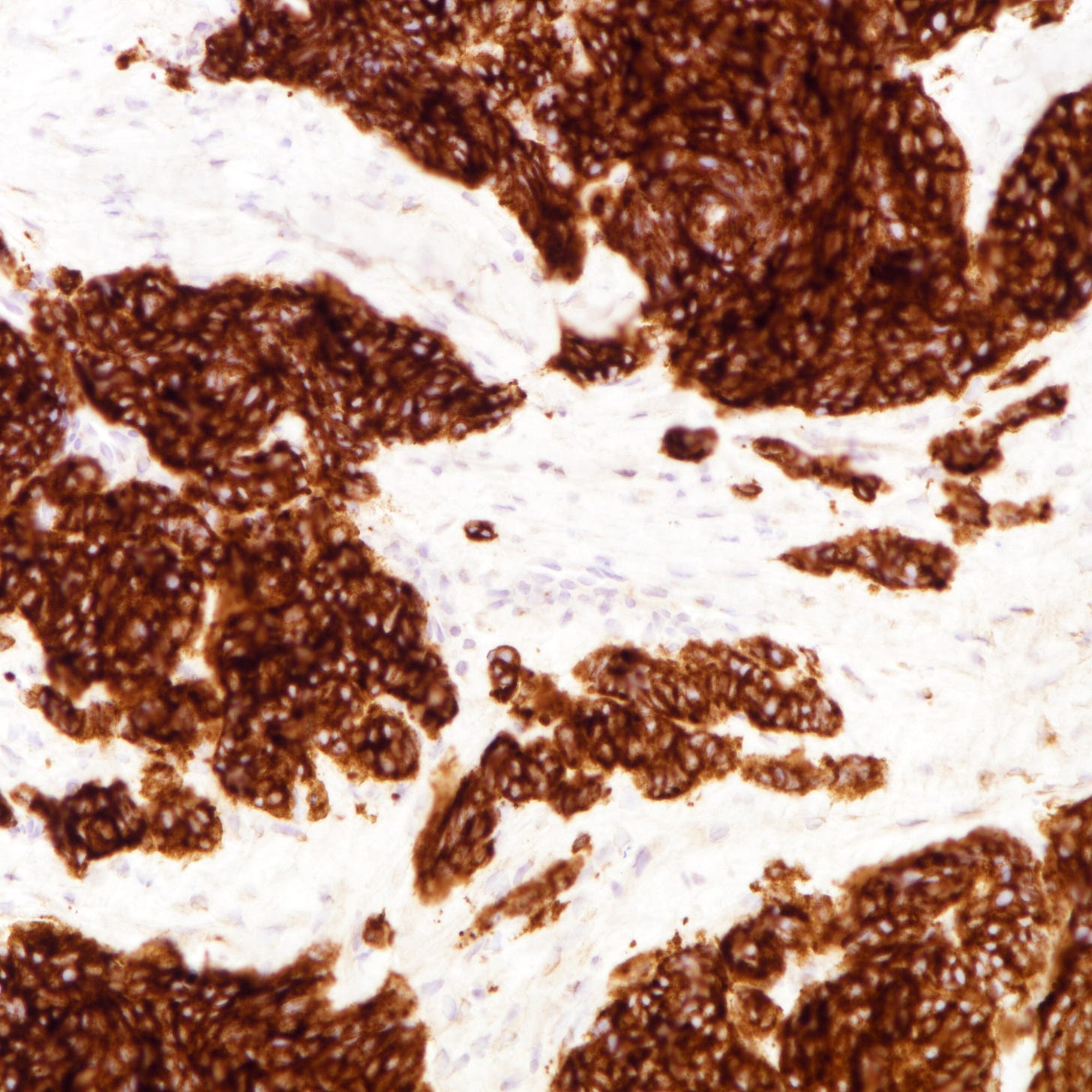
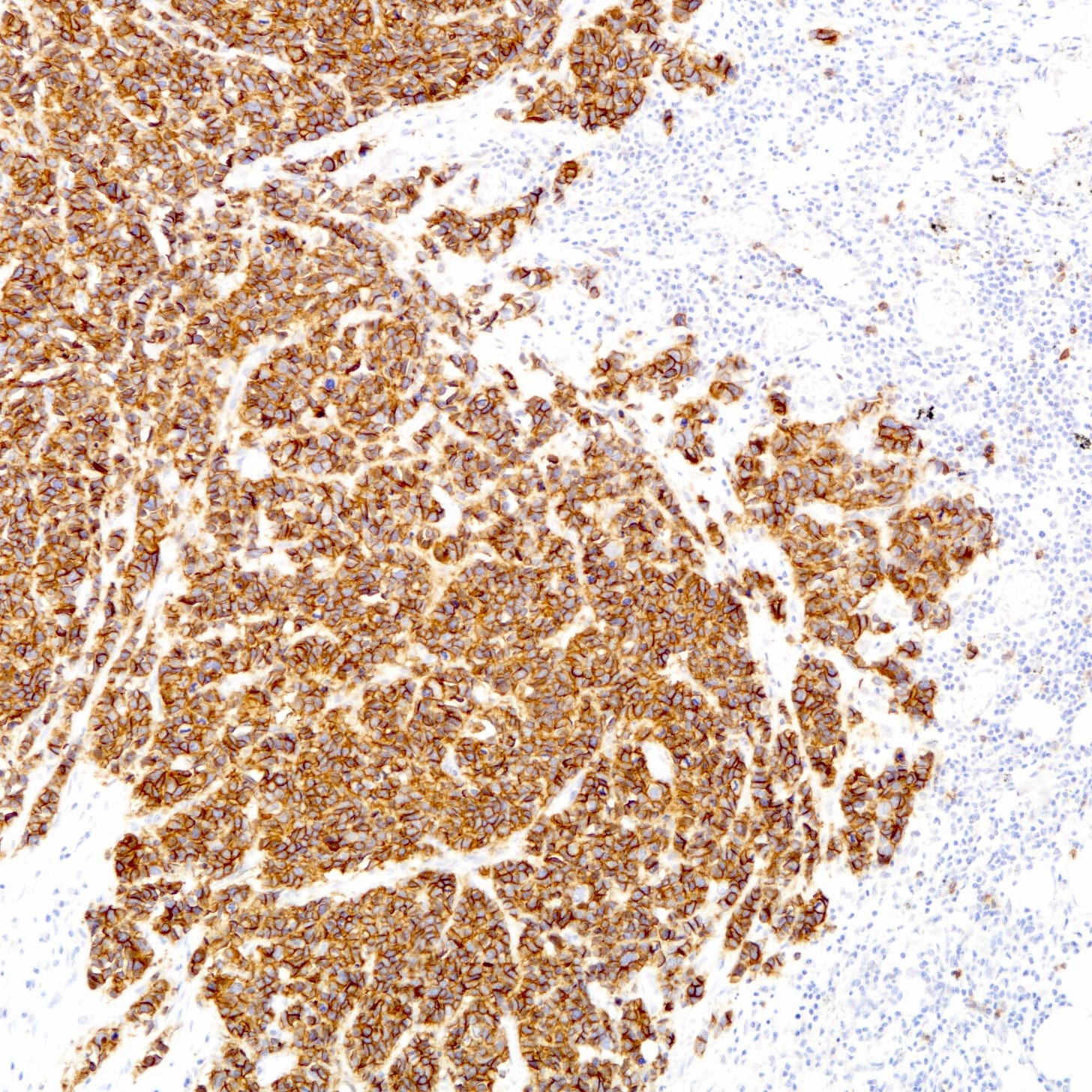
Immunohistochemically, neuroendocrine carcinomas express neuroendocrine markers including synaptophysin, chromogranin, CD56, INSM1 and neuron specific enolase. They are often positive for estrogen receptor (30 - 67%) and progesterone receptor (13 - 56%), although expression may be reduced compared to well differentiated tumors. HER2 immunohistochemistry is typically negative.
TP53 mutations are common in both small cell and large cell carcinoma of the breast. PI3K / PIK3CA mutations occur in 33% of small cell cases.
Neuroendocrine carcinoma of the breast - microscopic and cytology images

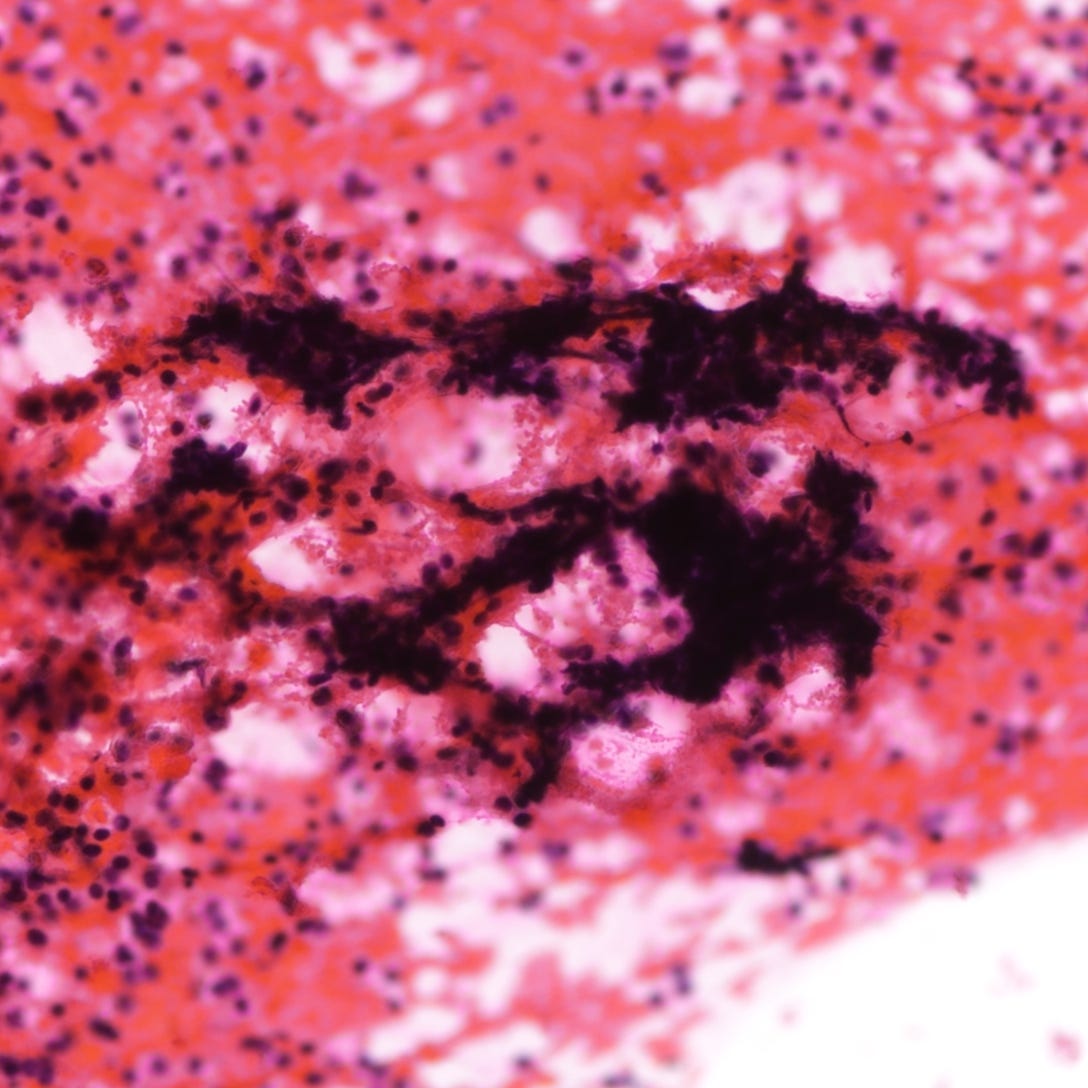
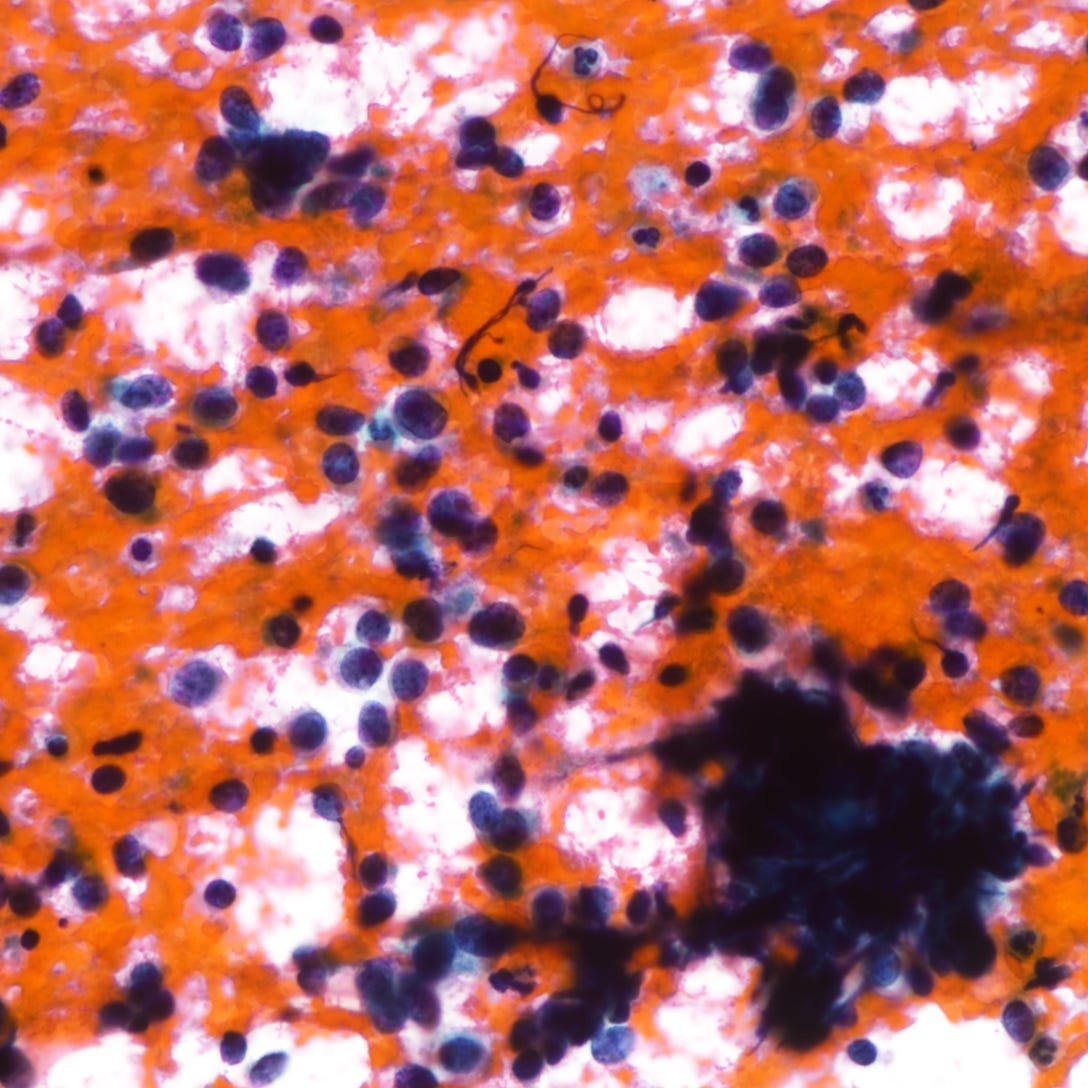
The next essay will discuss additional types of breast cancer that lack a known precursor.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me on LinkedIn, Threads and Instagram (@npernickmich) or Bluesky (@natpernick.bsky.social).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week, we post interesting cancer related images of malignancies with diagnoses, plus articles of interest. Please also read our CCN essays.
Latest cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email: Nat@PathologyOutlines.com (please note I cannot provide medical advice)
Follow my Substack Notes — subscribers are automatically notified.