Cancer precursor project - Premalignant precursors for glioblastoma
18 March 2024, revised 4 July 2025
The goal of our cancer precursor project is to better understand how cancer arises by compiling a regularly updated spreadsheet of all distinct human cancers (now ~1216) and their premalignant precursors (now ~178).
Malignant change is due to self-organized criticality, which describes catastrophic events such as earthquakes and stock market crashes, as well as malignant transformation. It is nature's way of making large changes based on individual factors thought too trivial to consider (Bak 1999). For example, in the punctuated equilibrium of species, one sees prolonged periods of apparent stasis (i.e., no new species), followed by bursts of new species (Eldredge & Gould 1972). During the “quiet” periods, minor changes are accumulating that may not be noticed. Similarly, under the influence of cancer risk factors or random events, human biological networks may have prolonged periods of minor changes with no apparent clinical or microscopic changes, followed by bursts of activity leading to premalignant precursors or frank malignancy (Cross 2016, Pernick 2023).
These premalignant precursors may be relatively stable based on the attractor concept and have distinctive molecular patterns that may or may not be identifiable histologically (Pernick 2018).
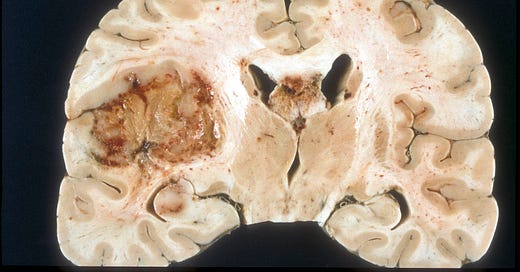
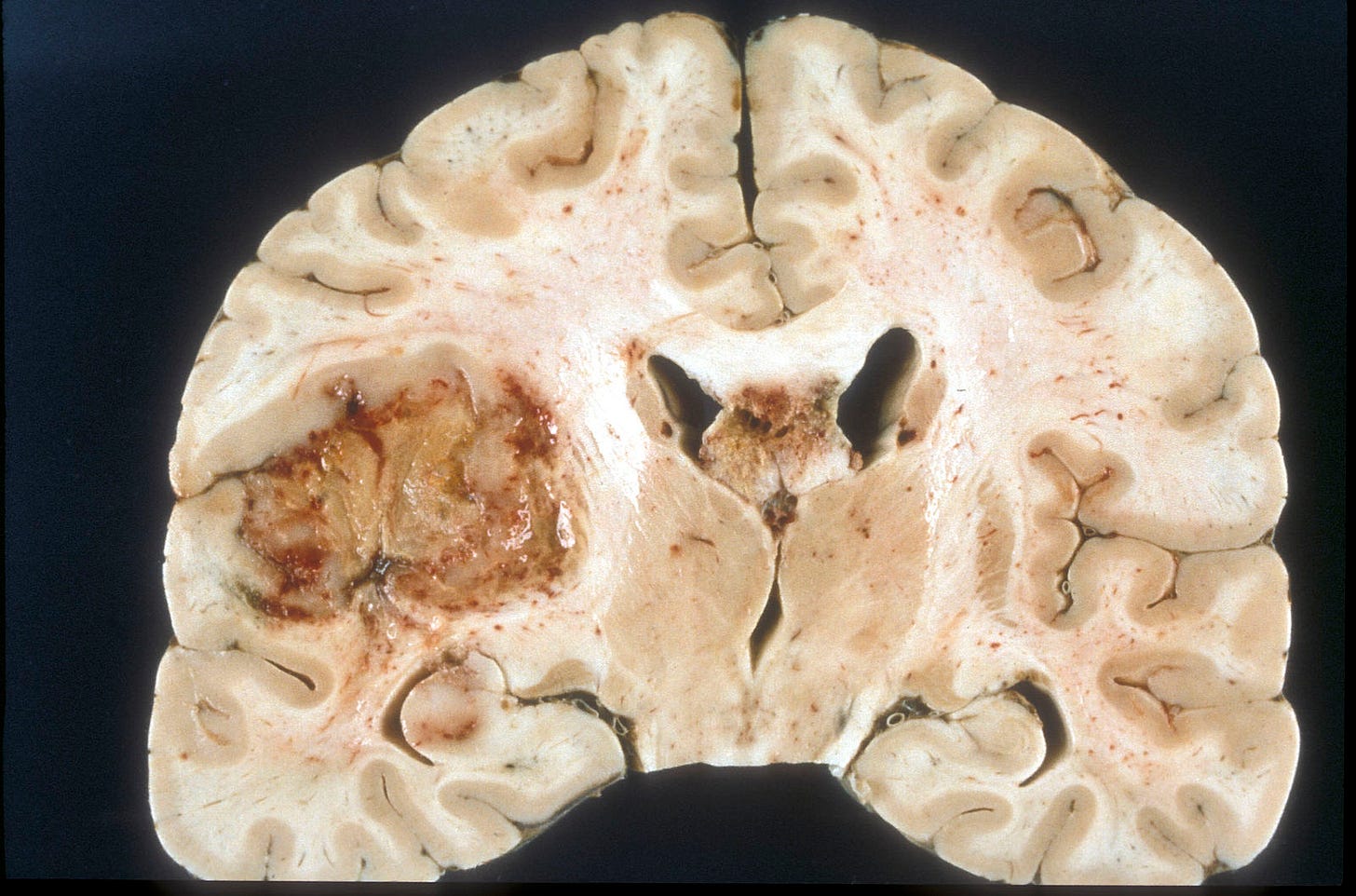
Glioblastoma, IDH wild type is the most common brain tumor in adults, excluding metastases. It accounts for 14% of all primary central nervous system (CNS) tumors and 49% of all malignant CNS tumors in adults (Ostrom 2021). Most glioblastomas are designated as primary (i.e., no evidence that it arose from a prior malignant lesion, Ohgaki 2013). They are aggressive tumors that typically occur in elderly patients with the rapid development of symptoms. Secondary glioblastomas arise from prior CNS tumors that are typically low grade, diffuse or anaplastic astrocytomas (see Hardian 2019 for an example). Secondary glioblastomas have different clinical and molecular features than primary glioblastomas: they occur in younger patients, more often in the frontal lobe, have a significantly better prognosis and have IDH1 mutations (Ohgaki 2013).
The 2021 World Health Organization (WHO) classification redefined glioblastoma as an adult grade 4 (aggressive) diffuse astrocytic glioma that is IDH and H3 wildtype (i.e., no mutations in these genes) and that has either: (a) microvascular proliferation or necrosis or (b) a TERT promoter mutation, EGFR gene amplification or +7/-10 chromosome copy number changes. The term glioblastoma multiforme is no longer used, and the term glioblastoma is no longer applied to pediatric tumors.
To date, no premalignant precursor lesion has been identified for primary glioblastoma or any primary CNS tumor (Pernick 2024). CNS malignancies may be initiated by a defining mutation in a single stem or progenitor cell or small cluster of cells that multiplies and acquires additional malignant properties over time. Due to its small size, detection may not be possible, at least with current methods, until it is large enough to be clinically evident.
Glioblastoma has three molecular subtypes based on gene expression profiling signatures: proneural, classical and mesenchymal, although this does not impact clinical practice, possibly due to marked intratumoral heterogeneity (i.e., tumor cells differ from each other) and the differentiation plasticity of glioblastoma (i.e., subtypes can change over time and through therapy, Ah-Pine 2023, Neftel 2019).
Glioblastoma may arise from neural stem cells or glial precursor cells by activating oncogenic pathways (Ah-Pine 2023). They may also originate from perivascular mesenchymal stromal cells which derive from the neural crest (Ah-Pine 2023).
If it exists, what might a glioblastoma premalignant precursor look like? We suggest it may have “milder” features of glioblastoma but in a nonmalignant context.
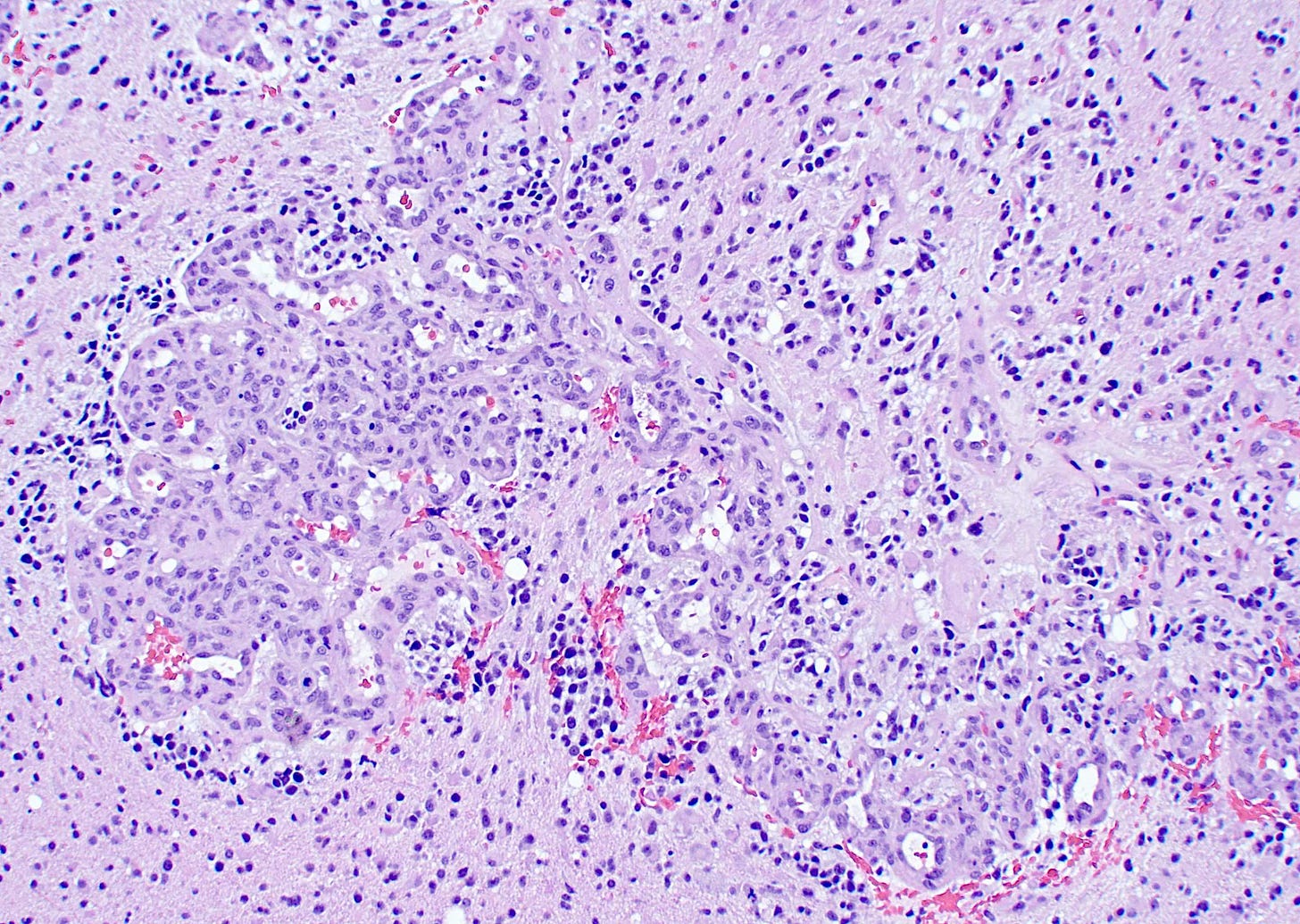
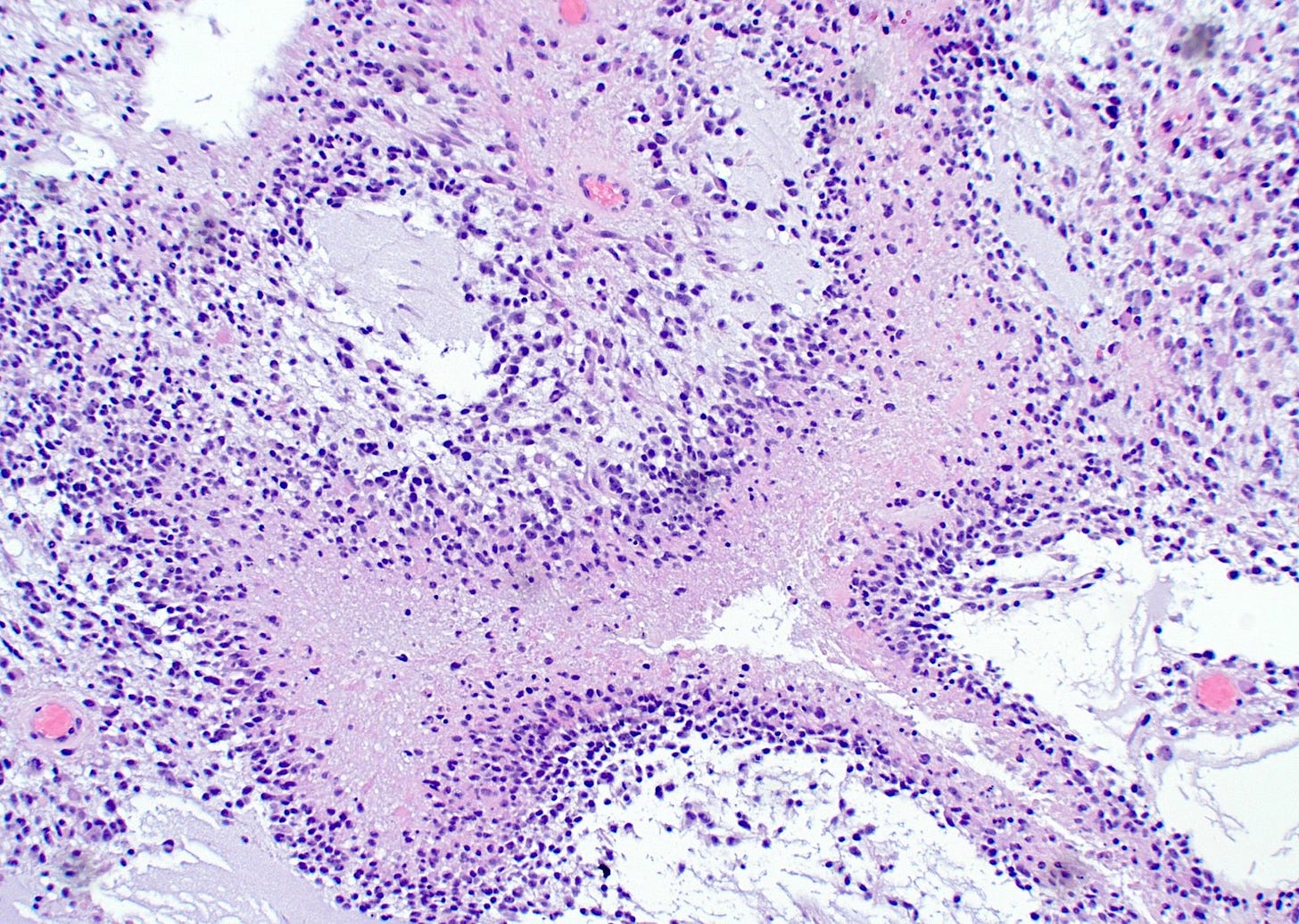
First, it may have microvascular proliferation, which consists of multilayered, small caliber vessels lined by endothelial cells and hyperplastic vascular smooth muscle cells creating a glomeruloid appearance (Haddad 1992). A normal glomerulus in the kidney is shown below followed by a glomeruloid microvascular proliferation in glioblastoma.

Since the prominent vasculature is an important component of glioblastoma, it may be part of its premalignant precursor. This vasculature is an intricate, multifaceted phenomenon that promotes tumor growth and spread by the following mechanisms (Mosteiro 2022):
Angiogenesis (the formation of new blood vessels from existing vessels via sprouting): this promotes tumor growth because tumor cells need oxygen and other nutrients supplied by blood.
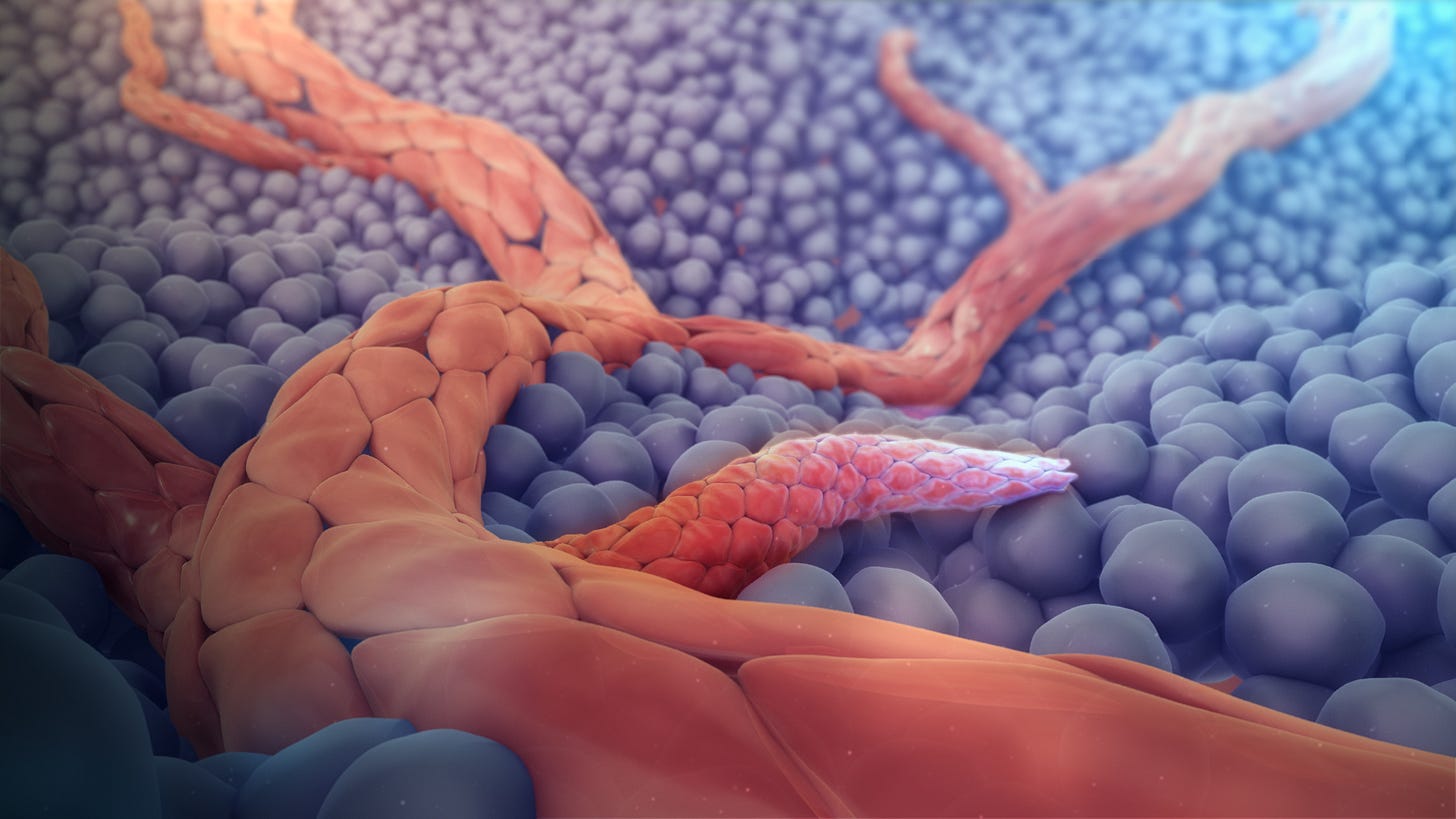
3D medical animation still showing angiogenesis, source Wikipedia Vasculogenesis (forming new blood vessels from precursor cells): endothelial cell progenitors are recruited from the bone marrow to assemble new blood vessels.
Transdifferentiation: glioblastoma stem cells differentiate into endothelial cells.
Vascular mimicry: glioblastoma stem cells differentiate into vascular smooth muscle cells or pericytes, which are components of blood vessels.
Vessel co-option: tumor cells migrate along existing blood vessels, enabling invasion throughout the brain, limiting surgical and even radiological detection of the tumor (Seano 2020).

Vascular generation and related processes: (A) angiogenesis, (B) vasculogenesis, (C) vascular mimicry, (D) vascular co-option (Mosteiro 2022, figure 2).
Microvascular proliferation in glioblastoma, particularly thick, branching and glomeruloid vessels, is also found in brain metastases of other tumor types (Gi 2017). Although glioblastomas only rarely metastasize (Lun 2011), microvascular proliferation may promote the malignant progression of glial cells in an unknown manner and may be an important feature of a premalignant precursor.
Second, a premalignant precursor to glioblastoma may have similar features to a low grade glioma, which is a precursor for secondary glioblastomas (Ohgaki 2013). Glioma stem cells can generate vascular pericytes that surround blood vessels within glioblastoma, so a premalignant glioblastoma precursor may also have prominent pericytes with this capability (Das 2013, Cheng 2013).
Third, a premalignant precursor to glioblastoma may have some type of necrotic niche, where hypoxia and lack of nutrients drive the formation of new vessels by diverse processes (Mosteiro 2022). Histologically, this may appear as focal areas of necrosis that are not associated with identifiable tumor cells.
A glioblastoma precursor may resemble embryonic or juvenile cells of the cerebrum. Mouse models have shown that glioblastoma cells resemble these cells, suggesting either the persistence of these early lineages following oncogene activation or their reactivation during malignant transformation (Hamed 2022).
Finally, glioblastoma precursor cells may have the propensity to produce the above features in the future, based on molecular or other changes, even if no histologic premalignant lesion is currently identified.
Autopsy studies
We suggest that premalignant precursor lesions be investigated using autopsy studies of glioblastoma patients (Griffin 2021), examining nontumor tissue for either the histologic changes noted above (microvascular proliferation, prominent perivascular pericytes, focal necrosis or embryonic differentiation) or for one of the defining molecular changes of glioblastoma in nontumor tissue (TERT promoter mutation, EGFR gene amplification or combined whole chromosome 7 gain and whole chromosome 10 loss) or to detect the overexpression of vascular endothelial growth factor (VEGF), an important mediator of angiogenesis in glioblastoma (Weathers 2015). Other possible common alterations of interest include those of the RTK genes, PI3K pathway genes and PTEN, present in up to 90% of glioblastomas (Brennan 2013).
The identification of premalignant precursors of glioblastoma may provide a better understanding and ultimately more effective treatment.
If you enjoy these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me on LinkedIn, Threads and Instagram (@npernickmich) or Bluesky (@natpernick.bsky.social).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or in the CCN section of our PathologyOutlines.com blog. Each week, we post interesting cancer related images of malignancies with diagnoses, plus articles of interest. Please also read our CCN essays.
Latest cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email: Nat@PathologyOutlines.com (please note I cannot provide medical advice)
Be sure to follow my Substack Notes — subscribers are automatically notified.