Cancer precursor project - breast cancer, part 6d
30 December 2024, revised 4 February 2025
In part 6a of our cancer precursor project, we discussed breast cancer mortality, breast cancer risk factors and how breast cancer arises. In part 6b, we discussed breast anatomy and histology and three breast cancers with known precursors: infiltrating duct carcinoma of no special type, lobular carcinoma and pleomorphic lobular carcinoma. We also discussed their precursors: ductal carcinoma in situ (DCIS), classic lobular carcinoma in situ (LCIS) and its variants of florid and pleomorphic LCIS, as well as atypical lobular hyperplasia. In part 6c, we discussed 4 other types of breast carcinoma with ductal carcinoma in situ (DCIS) as a precursor: cribriform carcinoma, secretory carcinoma, neuroendocrine-small cell carcinoma and male invasive breast carcinoma.
In part 6d, we discuss four more breast cancers with known precursors: tubular carcinoma (precursors are flat epithelial atypia and DCIS-low grade), triple negative breast cancer (precursor is microglandular adenosis), acinic cell carcinoma (precursor is also microglandular adenosis) and metaplastic carcinoma: squamous cell carcinoma (precursor is squamous metaplasia).
Tubular carcinoma of the breast
Tubular carcinoma of the breast is an uncommon, well differentiated, invasive breast cancer that comprises 2% of all invasive breast carcinomas.
It tends to occur in postmenopausal women with a median age at presentation of 63 years. Forty percent of patients have a family history of breast carcinoma. Most patients present with early stage, pT1 and pN0 disease, which is multifocal in 20% of cases.
Tubular carcinoma has an excellent prognosis, similar to DCIS (97% cause specific survival at 10 years), but only if it lacks a component of invasive ductal carcinoma of no special type (a contrary view is here). Surprisingly, axillary nodal metastases are found in 10- 27%, even in patients with tumors 1 cm or less, but these patients still have an excellent prognosis.
Treatment consists of surgery with radiotherapy. Patients may be offered anti-endocrine therapies. Patients typically do not receive adjuvant chemotherapy and the use of axillary lymph node staging is disputed (needed, not needed).
Grossly, the tumor is ~1 cm, spiculated in appearance with a firm consistency. It may have a gritty texture if there are calcifications.
Microscopically, > 90% of the tumor is composed of an infiltrative growth pattern of well differentiated, ovoid or angular tubules with open lumina lined by a single layer of epithelial cells (i.e. there is no myoepithelial layer) with low grade nuclei and sparse mitoses (grade 1). Tumor cells often have apical cytoplasmic tufting or snouts. There is a fibrous or desmoplastic stromal response. Tumor cells are ER positive and HER2 negative.
A three dimensional modeling study revealed that the tumor structure resembles a necklace with the appearance of the “tubules” related to the plane of sectioning.
Tubular carcinoma is frequently associated with and appears to arise from columnar cell lesions (95%), in particular flat epithelial atypia, as well as atypical ductal hyperplasia and low grade DCIS.
Tubular carcinoma of the breast may arise from epigenetic abnormalities that lead to inactivation of tumor suppressor genes, DNA repair genes, cell cycle regulators and transcription factors. Chromosomal aberrations, especially 16q loss, occur over the spectrum of flat epithelial atypia, atypical ductal hyperplasia, low grade DCIS and low grade breast carcinomas, including tubular carcinoma. The specific genes lost at 16q relevant to this low grade breast carcinoma pathway are still unknown. However, gene dosage transcriptional effects may play a role in tumorigenesis and cancer progression - the gene dosage compensation hypothesis proposes that the expression of certain genes is altered to compensate for differences in the dosage of those genes when additional chromosomes are present, which helps cancer cells cope with the damaging effects of aneuploidy.
Tubular carcinoma of the breast - microscopic images
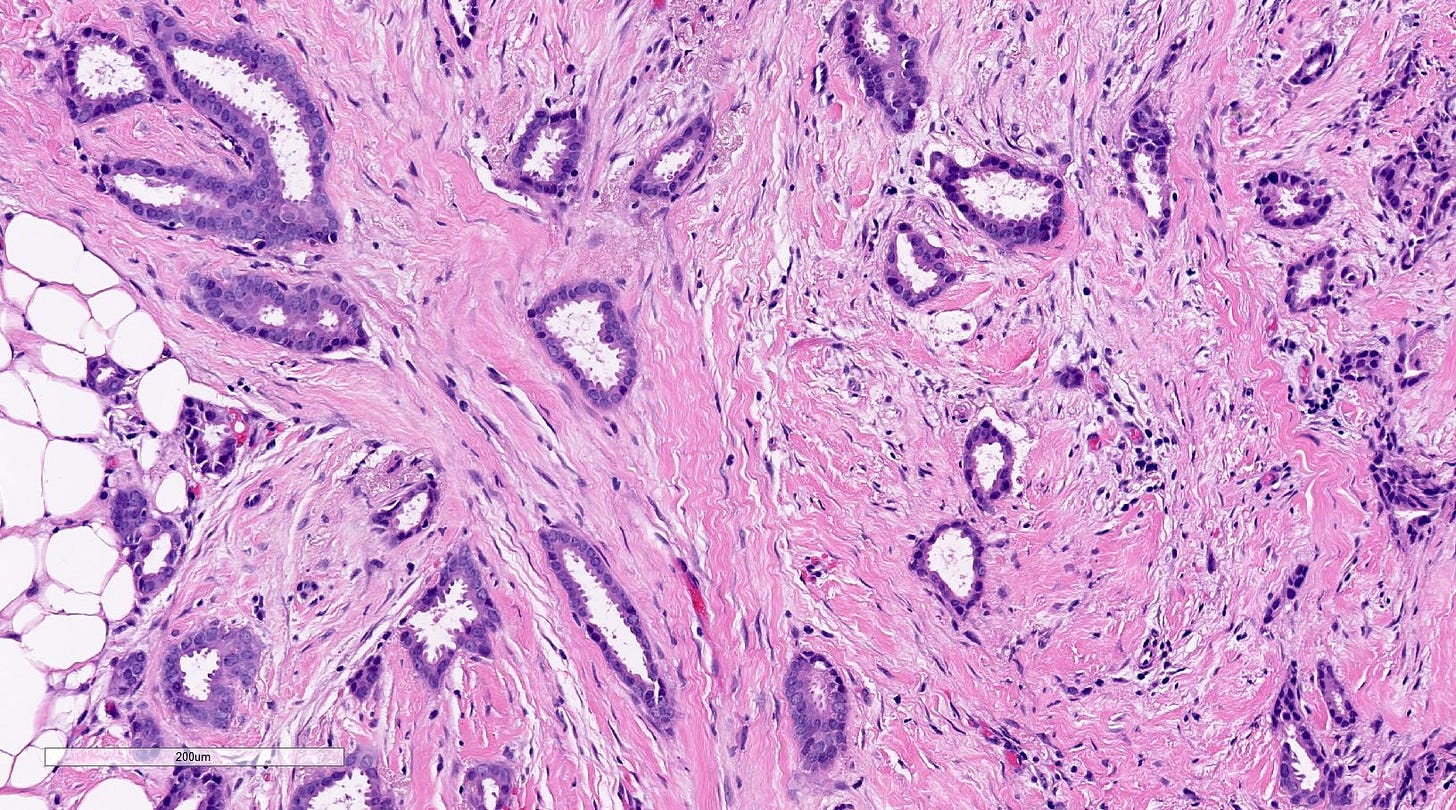
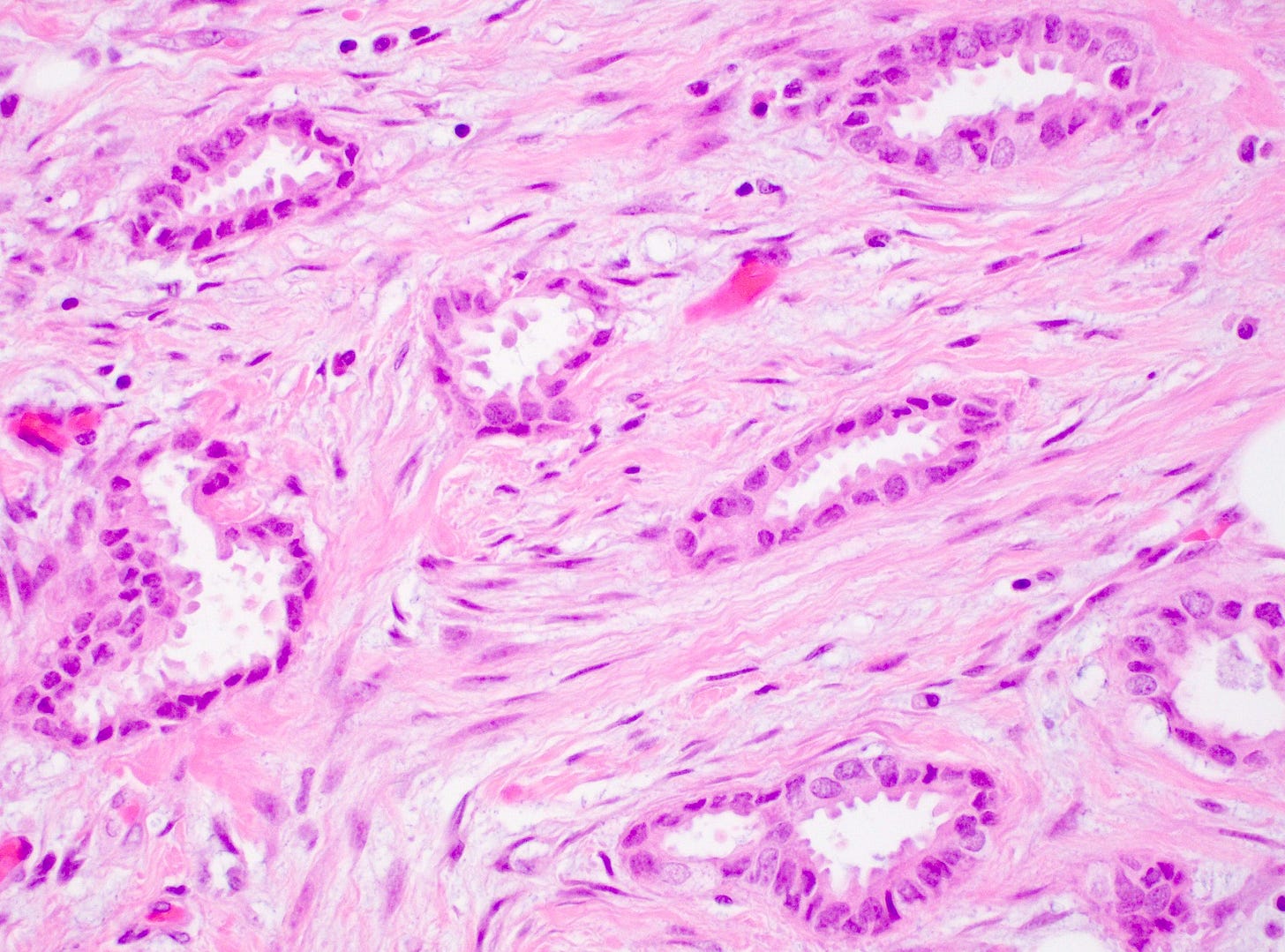
Precursor of tubular carcinoma - flat epithelial atypia
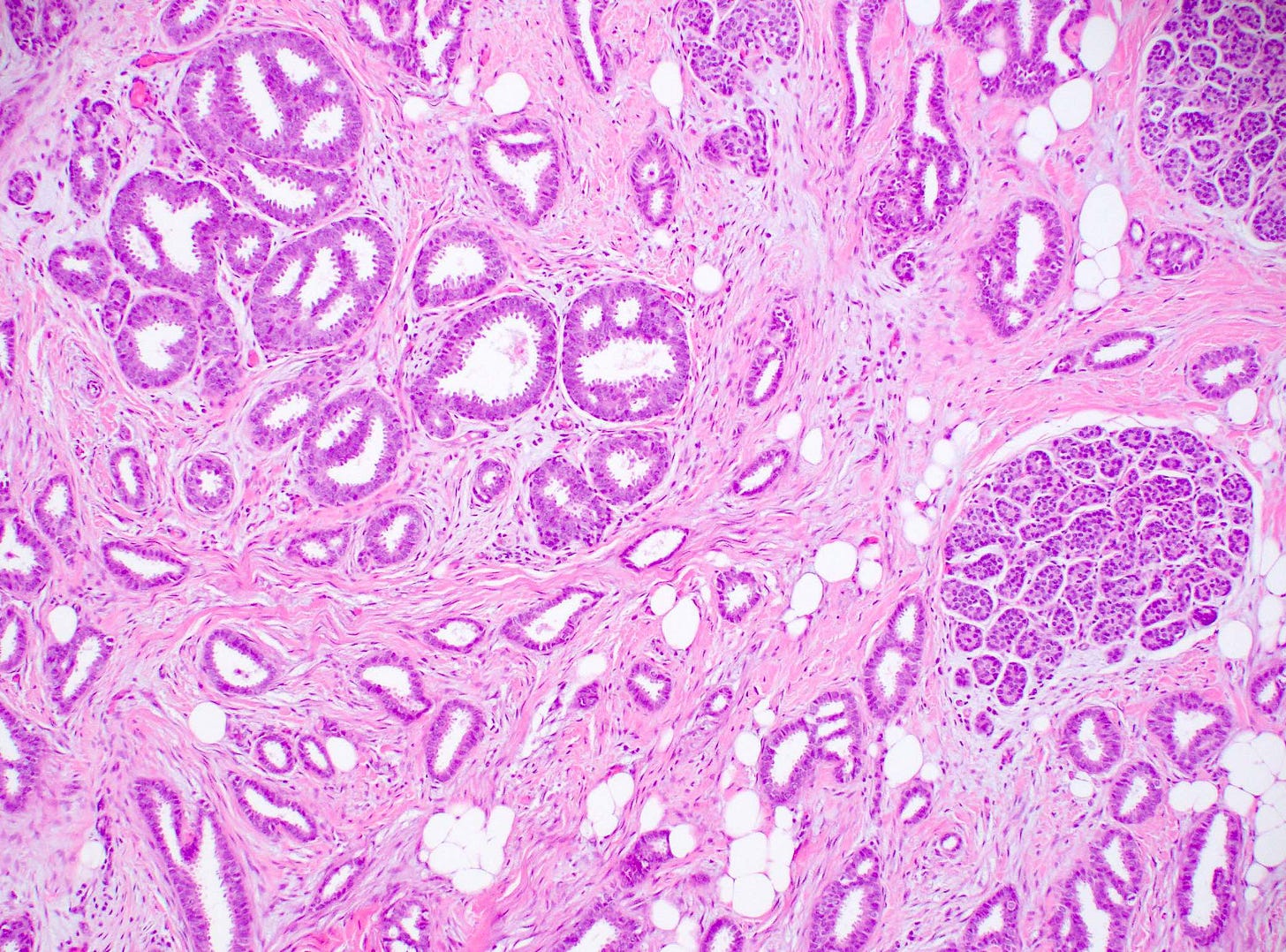
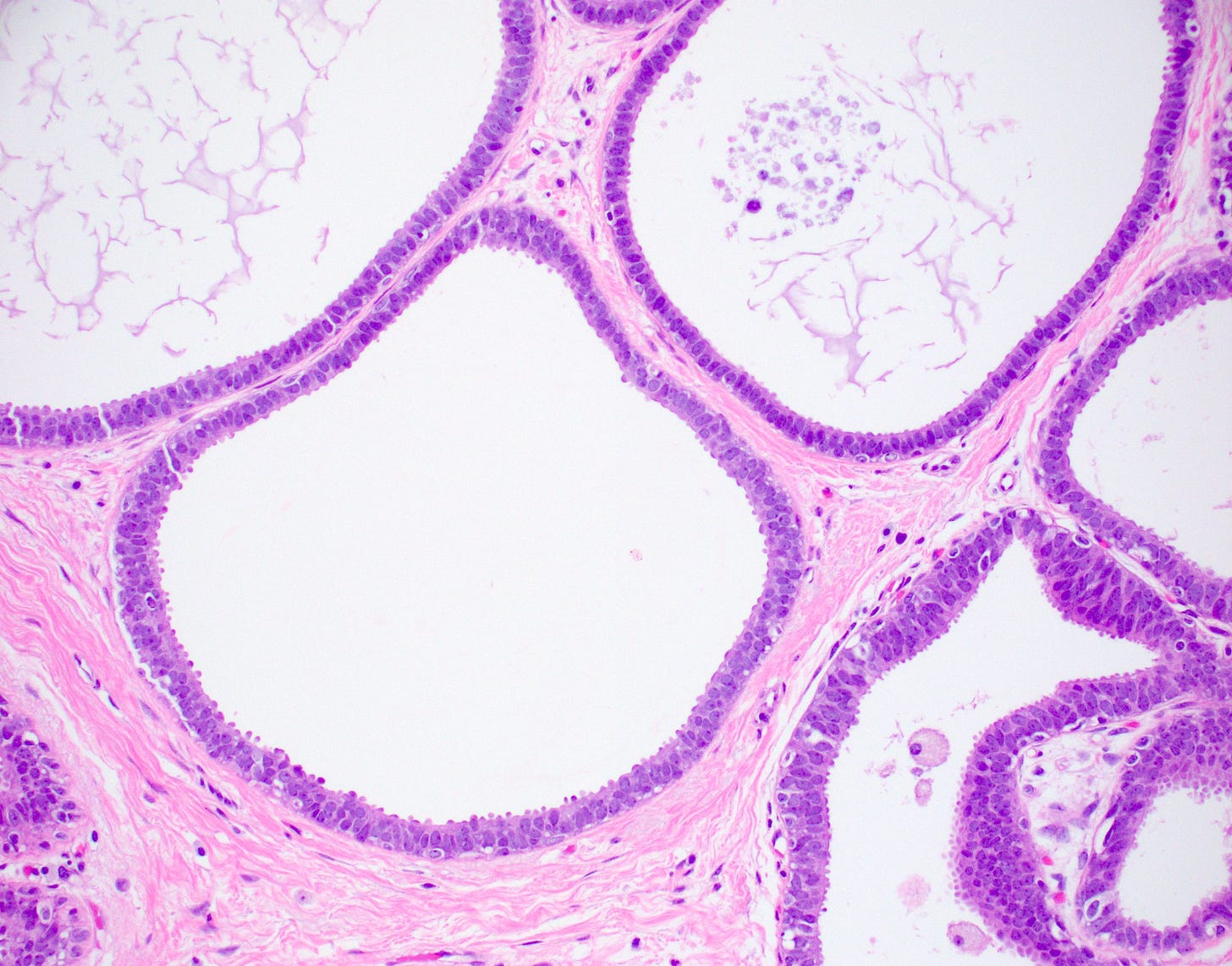
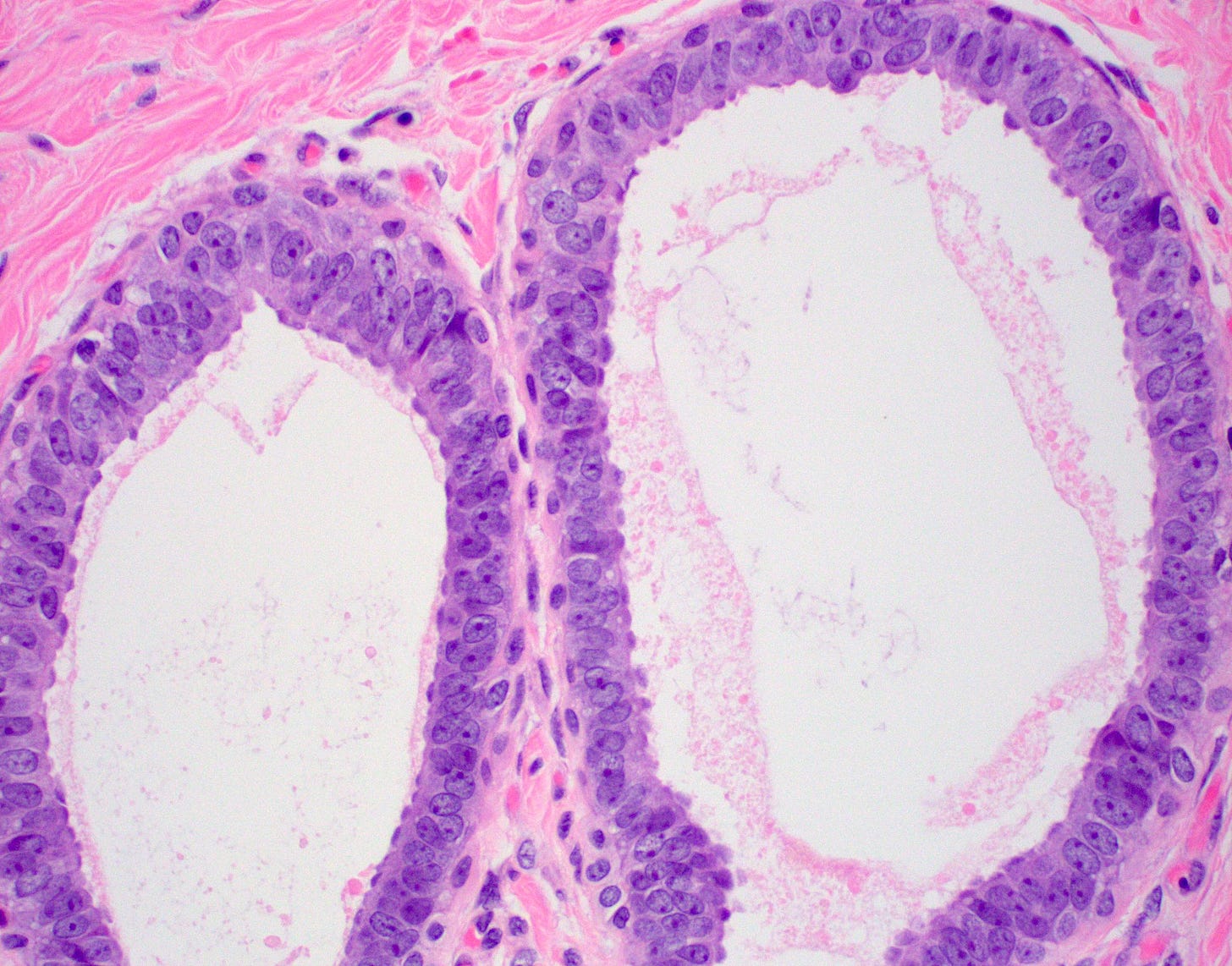
Flat epithelial atypia is considered a precursor of tubular carcinoma and other low grade breast carcinomas because they share molecular alterations and often coexist, although the risk of progression from flat epithelial atypia to frank malignancy is low. “Flat epithelial atypia” was introduced by the World Health Organization and encompasses columnar cell lesions with low grade or monomorphic cytologic atypia that lack architectural features of atypical ductal hyperplasia or low grade ductal carcinoma in situ. Specifically, it is an atypical epithelial proliferation with one to several layers of disorganized, monotonous, low columnar to cuboidal cells without architectural atypia.
Flat epithelial atypia is identified in 4 - 10% of breast core needle biopsies. It appears to be part of the low grade pathway in the development of breast cancer. If found, pathologists should look carefully for other components of this pathway such as atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ and low grade ductal carcinoma in situ.
Precursor of tubular carcinoma - Flat epithelial atypia - microscopic images
Triple negative breast cancer
Triple negative breast cancer is defined as an invasive breast cancer that does not show expression of estrogen receptor (ER), progesterone receptor (PR) or HER2 by immunohistochemistry. It represents 16% of invasive breast cancers.
Many breast cancer subtypes may be triple negative, including:
Basal-like breast cancers comprise about 80% of triple negative cancers but are actually defined by molecular (gene expression) profiling. Basal cell breast cancer is not considered a distinct subtype in the WHO classification of breast malignancies.
High grade (aggressive tumors): infiltrative ductal carcinoma of no special type grade 3, metaplastic breast carcinoma-spindle cell type, medullary carcinoma, apocrine carcinoma, BRCA associated carcinoma.
Low grade: salivary gland-like tumors (adenoid cystic carcinoma, acinic cell carcinoma [discussed below], mucoepidermoid carcinoma, secretory carcinoma), tall cell carcinoma with reverse polarity, metaplastic carcinoma including squamous cell carcinoma [discussed below], adenosquamous carcinoma and low grade fibromatosis-like types.
Triple negative carcinomas generally present with a high stage and high grade tumor that is larger and has a higher rate of nodal and distant metastases than infiltrative ductal carcinomas of no special type.
Triple negative carcinomas are more common in younger patients than other types of invasive breast carcinoma and are more common in African Americans than Caucasians. Although their cause is unknown, hormonal factors may play a role as the risk is increased in women with longer oral contraceptive use but the risk is decreased with later age at menarche, later age at first birth and breastfeeding.
Triple negative carcinomas constitute 71% of breast cancers in BRCA1 carriers compared to 25% of breast cancers in BRCA2 carriers and 16% of all invasive breast cancers.
The standard treatment for triple negative breast cancer is complete surgical excision. Neoadjuvant chemotherapy is given for locally advanced disease but is also recommended for early disease. Patients with metastatic / advanced / recurrent disease may receive PDL1 inhibitors or PARP inhibitors combined with chemotherapy, based on molecular testing.
Patients with triple negative breast cancer tend to have shorter survival and a higher risk of metastases. They do not benefit from anti-estrogenic therapy (since they are ER and PR negative). Patients with low grade disease have an excellent prognosis.
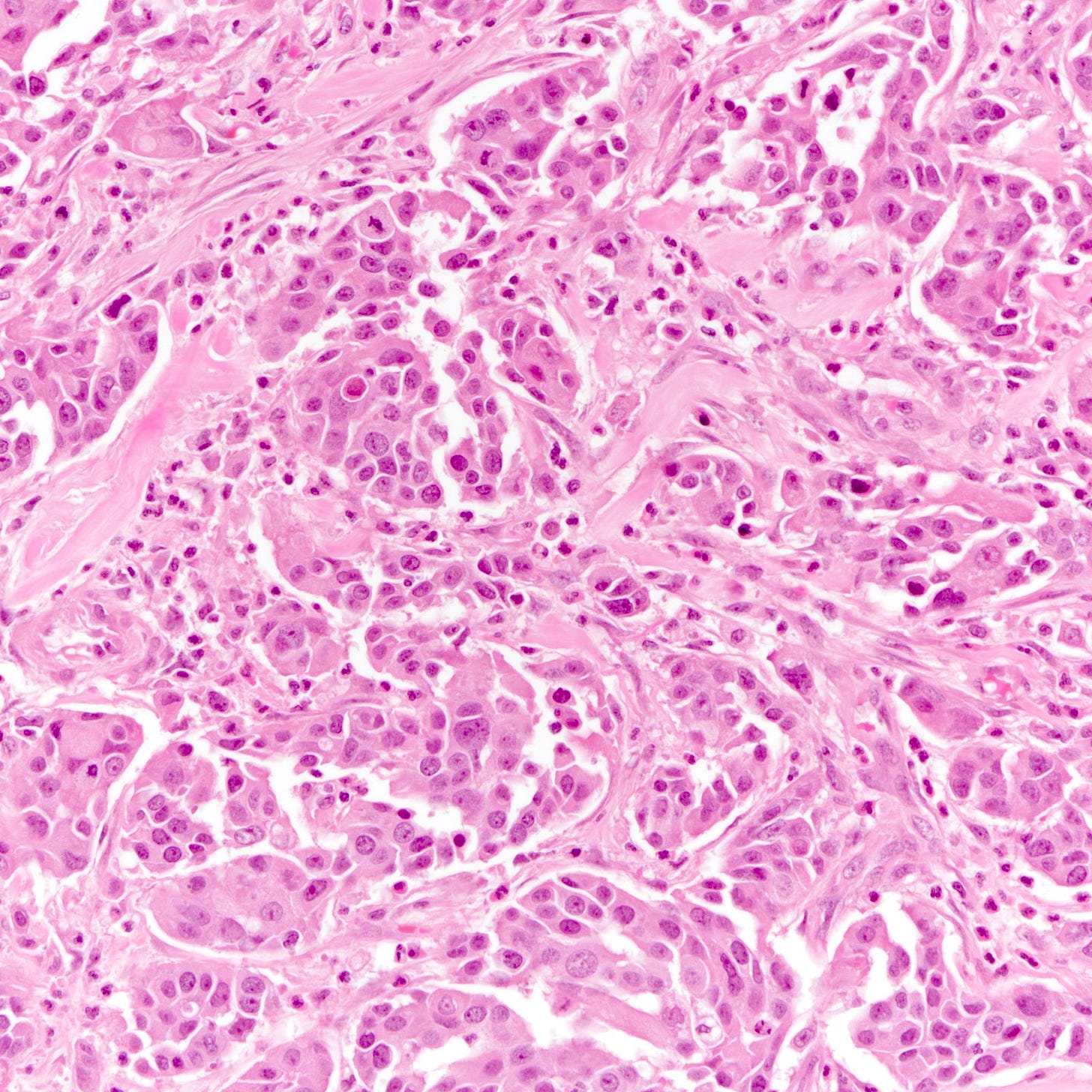
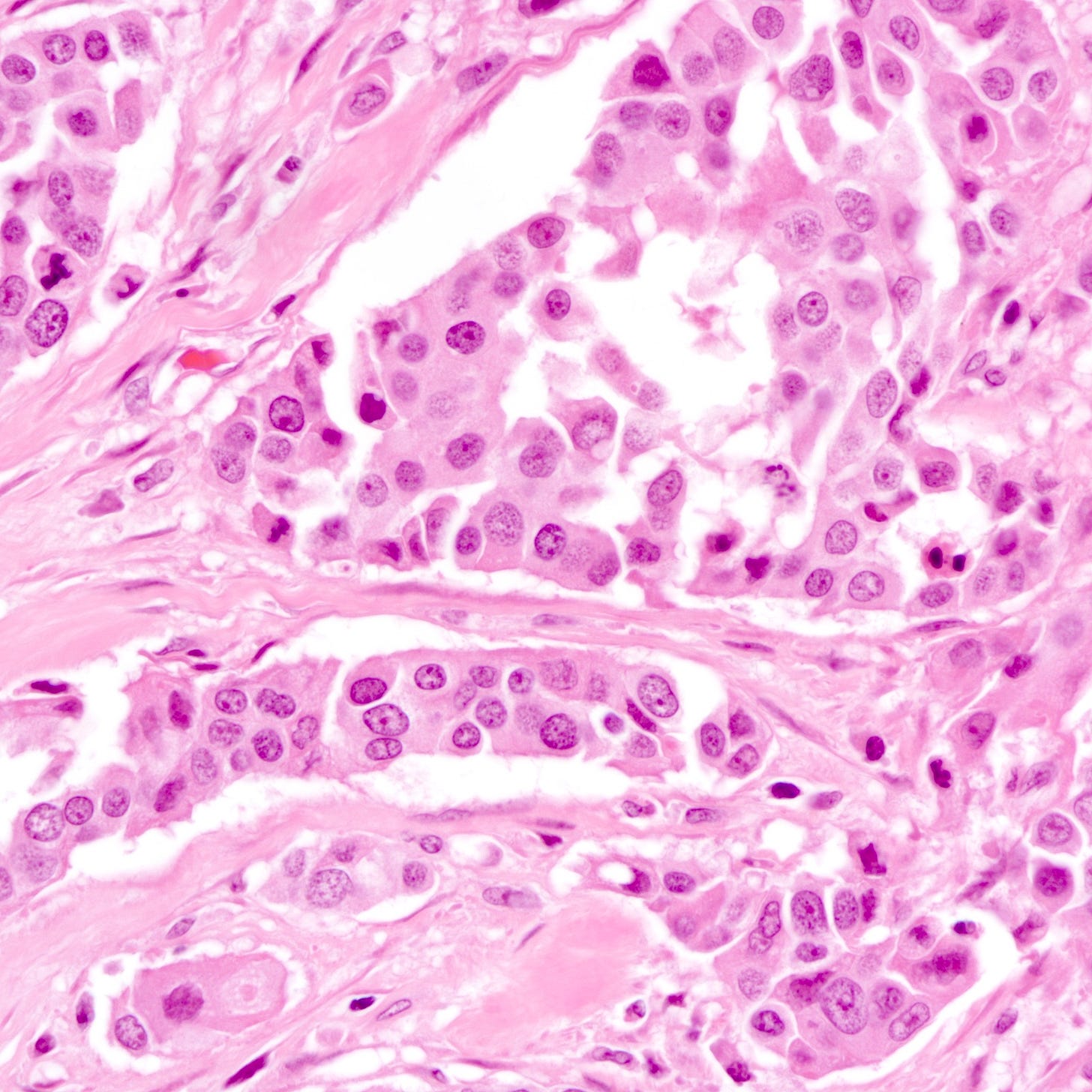
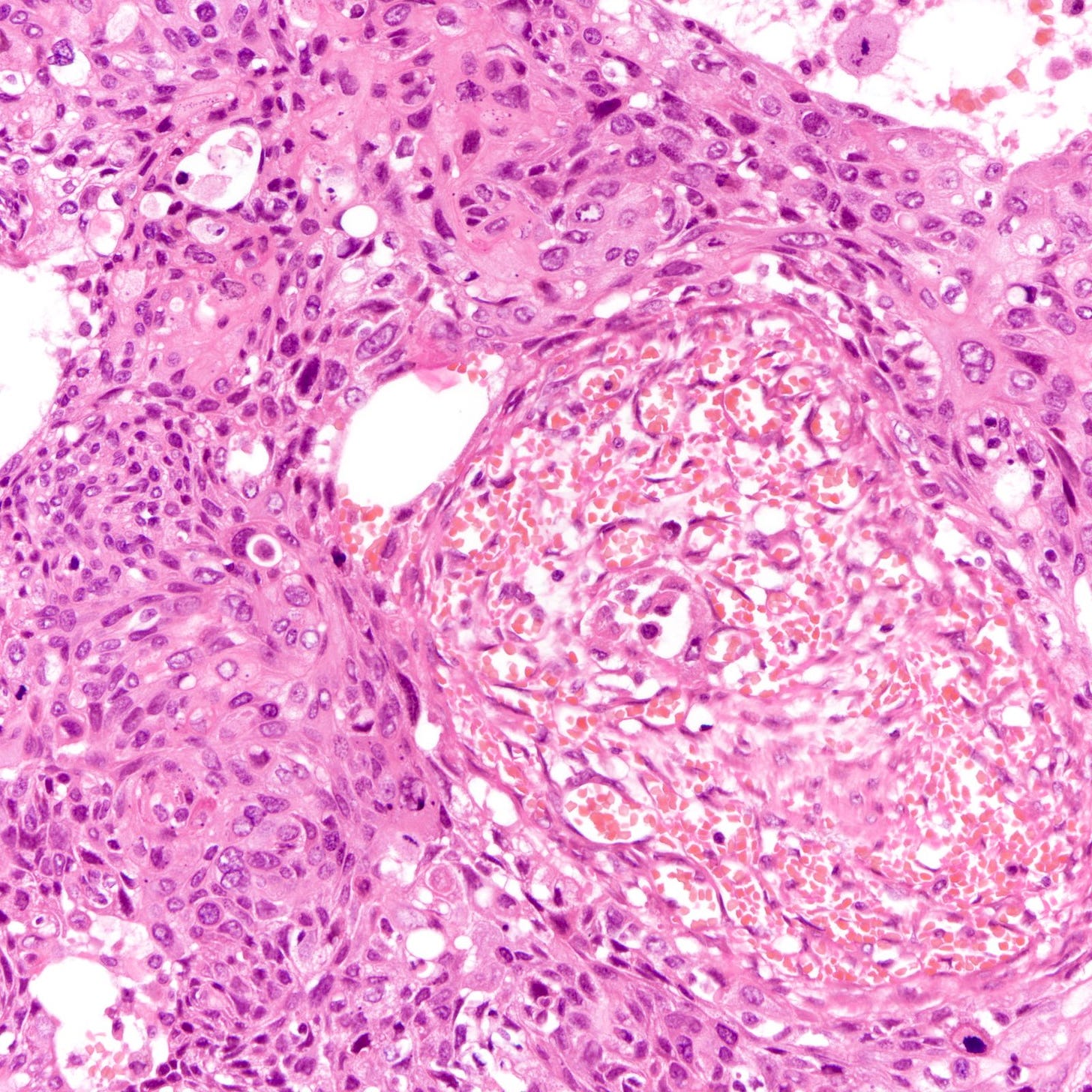
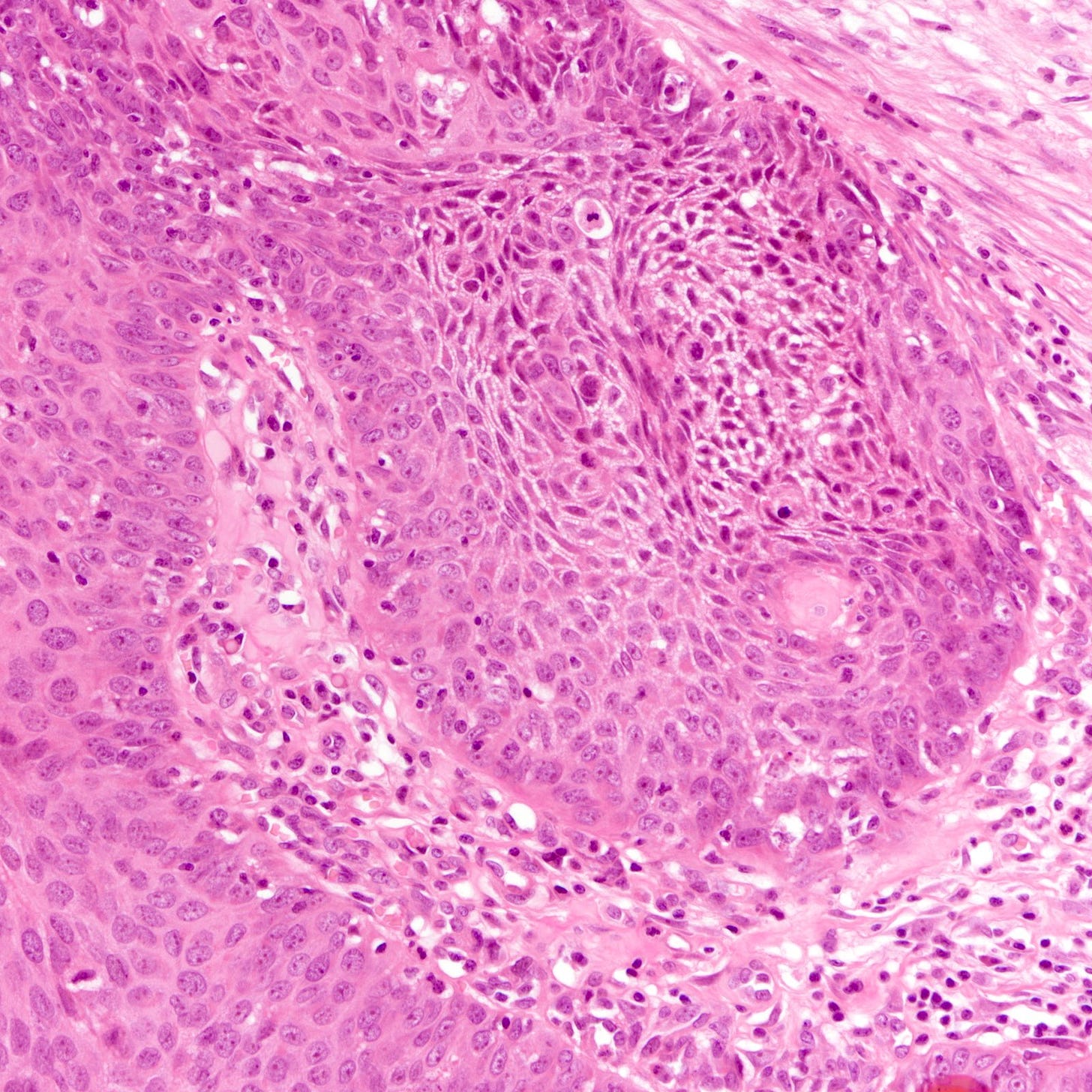
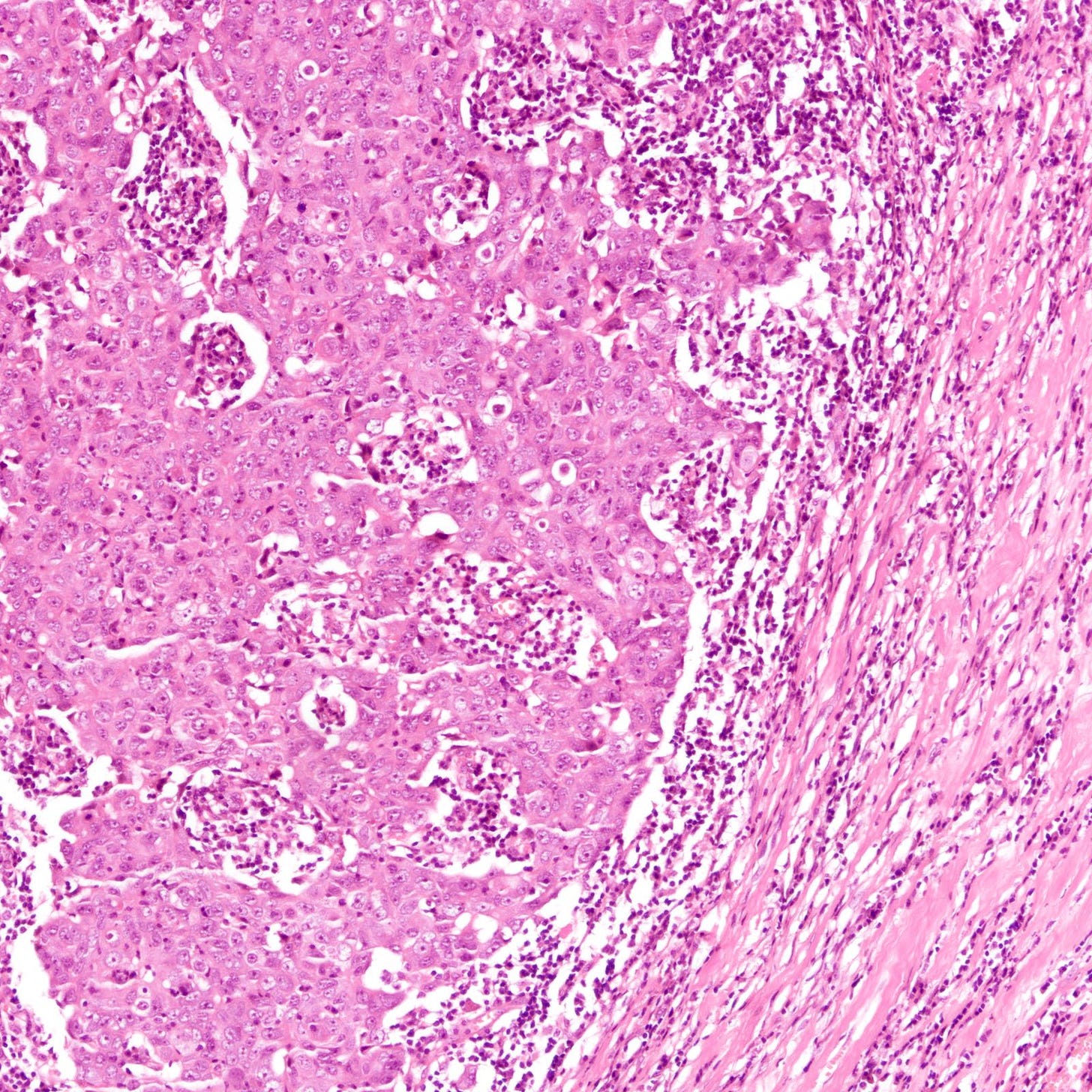
The histology of triple negative breast carcinoma is determined by the subtype present - being negative for ER, PR and HER2 by immunohistochemistry does not confer any distinct gross or microscopic appearance. Most triple negative breast carcinomas are invasive ductal carcinomas, no special type and are poorly differentiated or high grade (grade 3).
Triple negative breast carcinoma - microscopic images
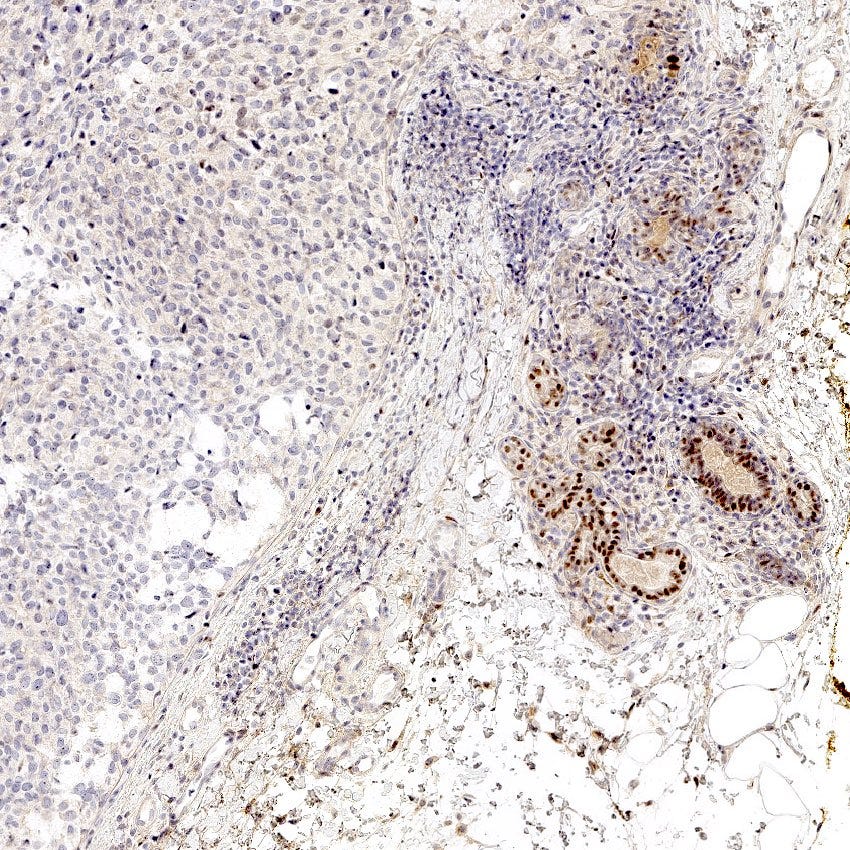
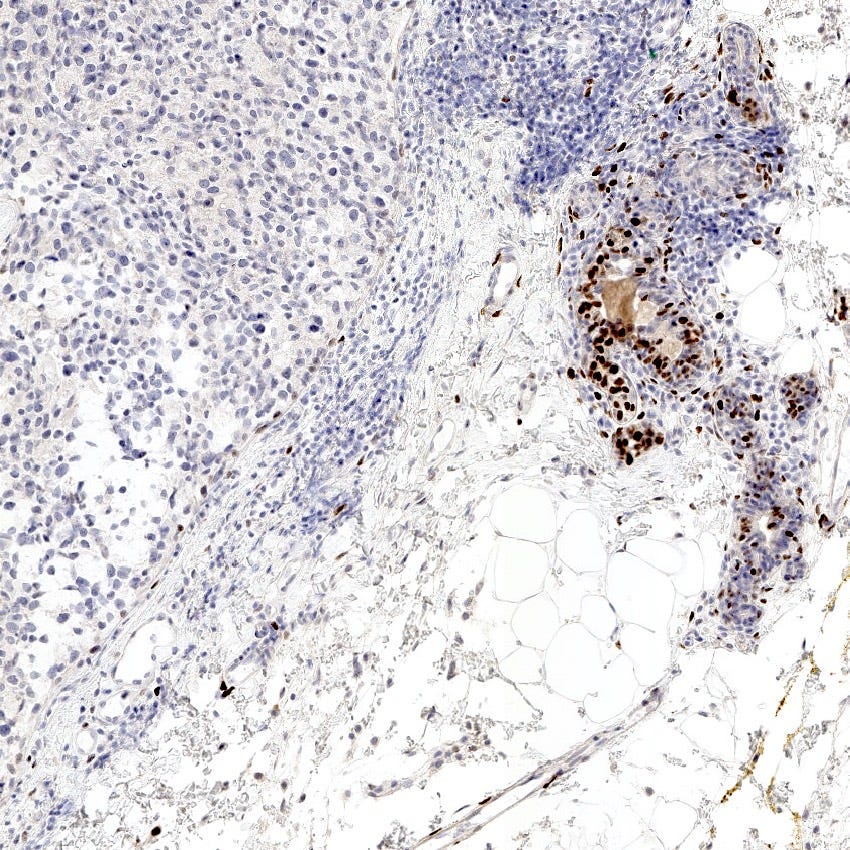
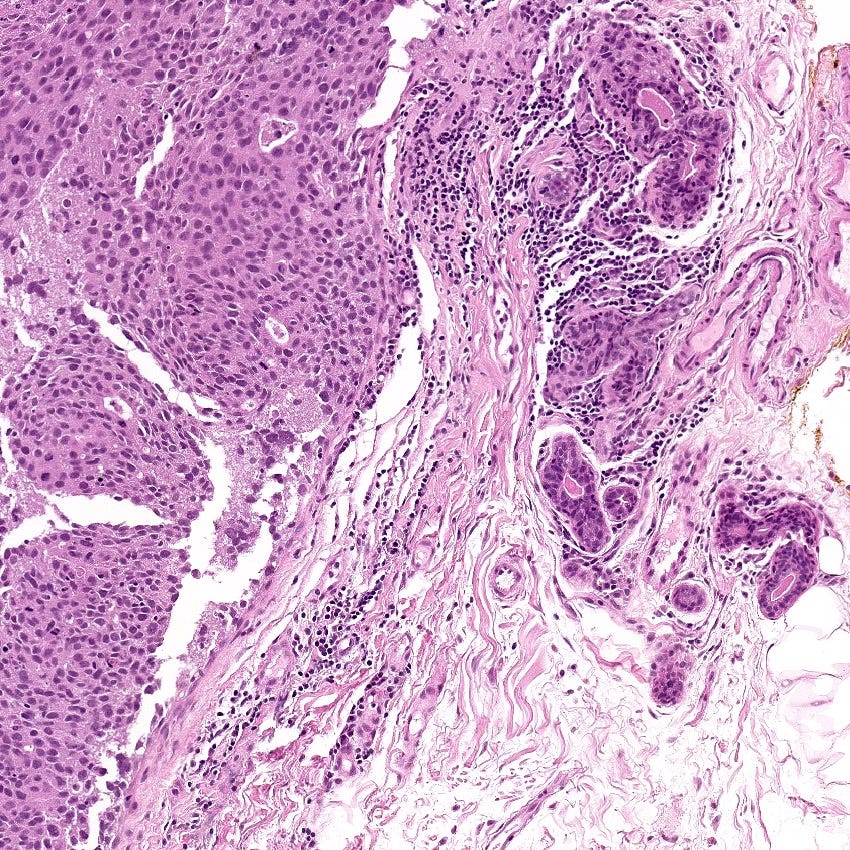
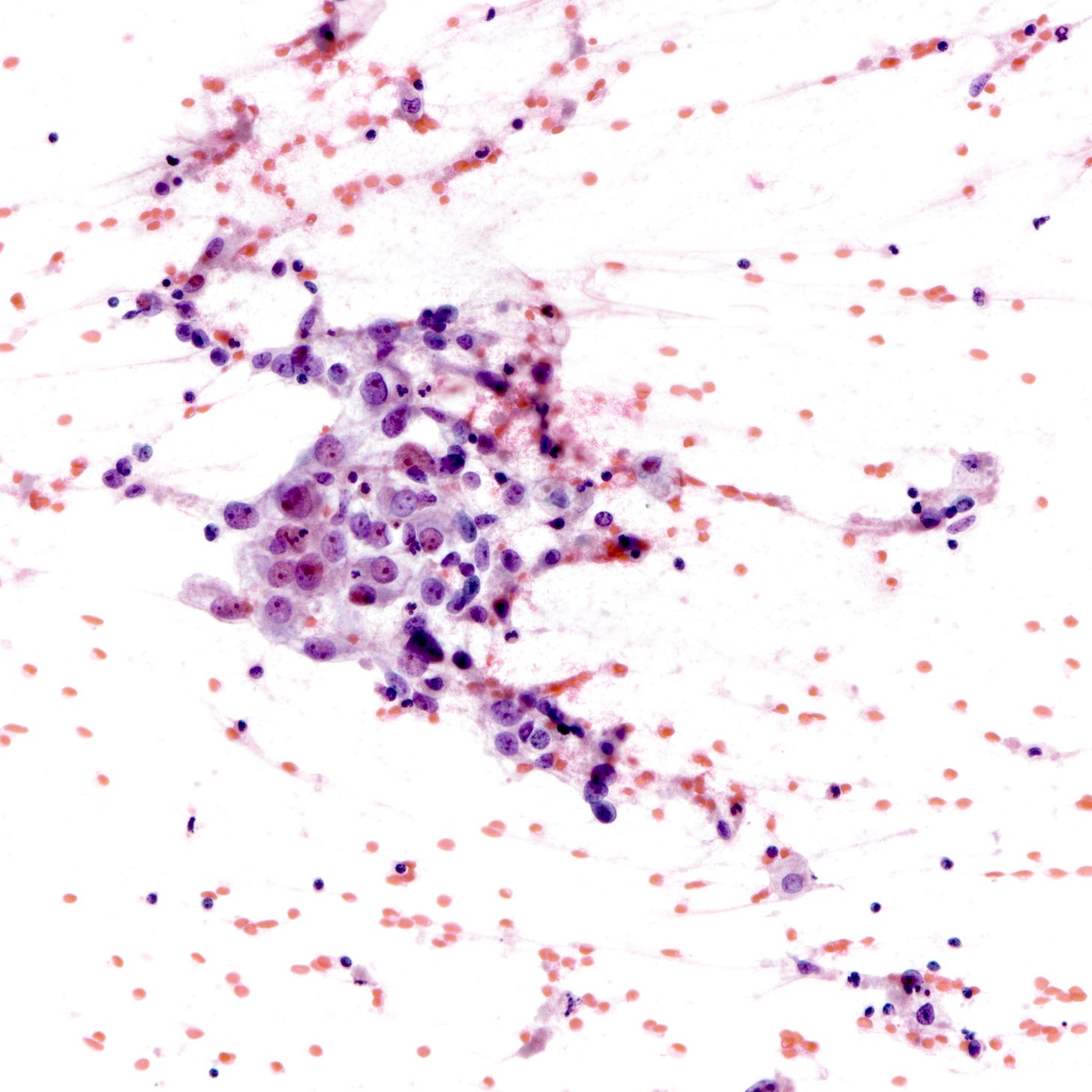
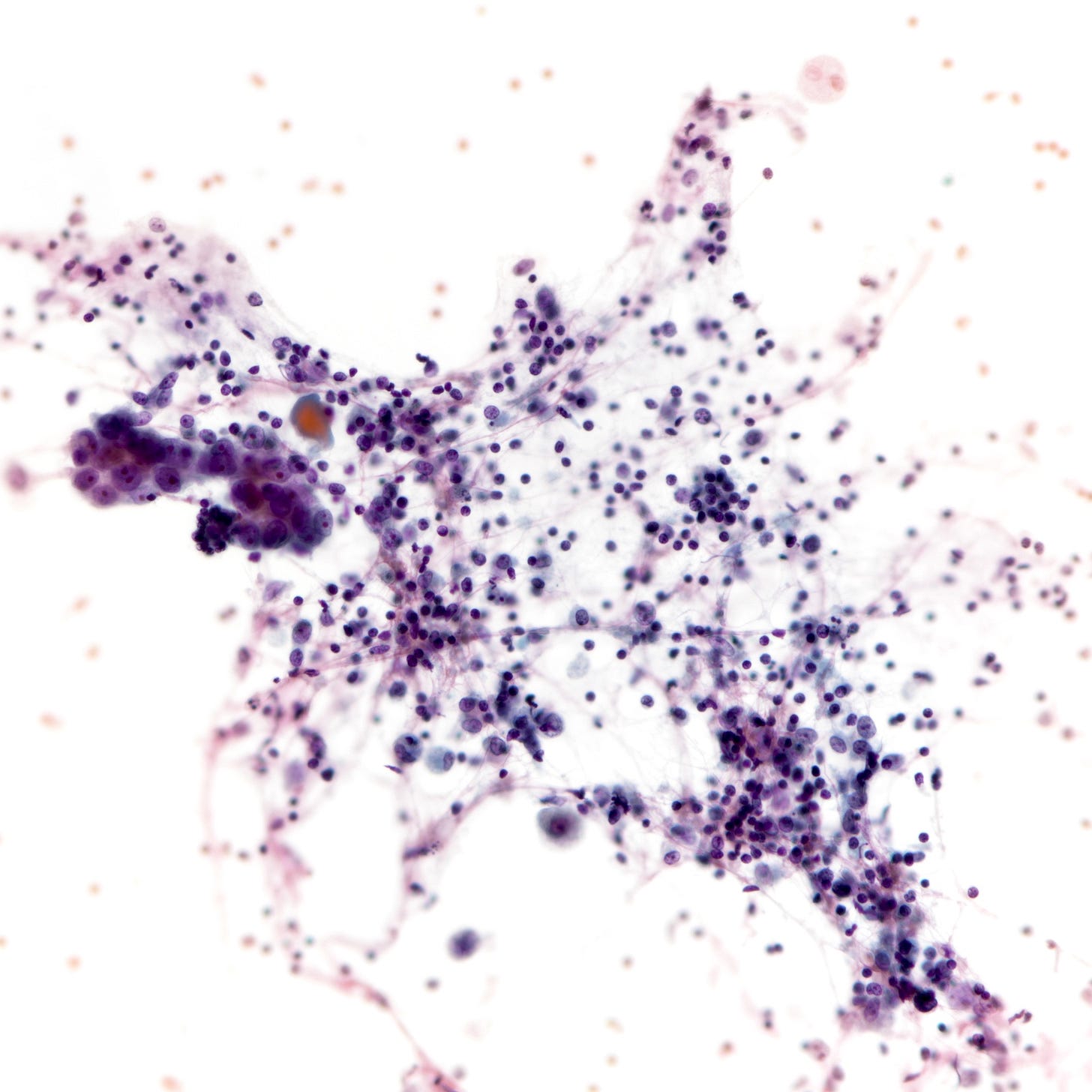
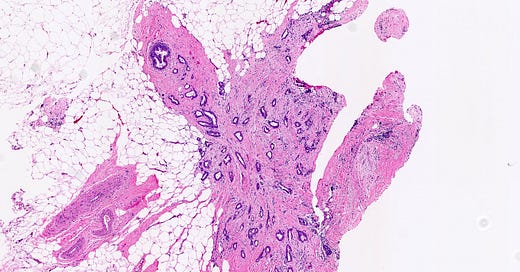
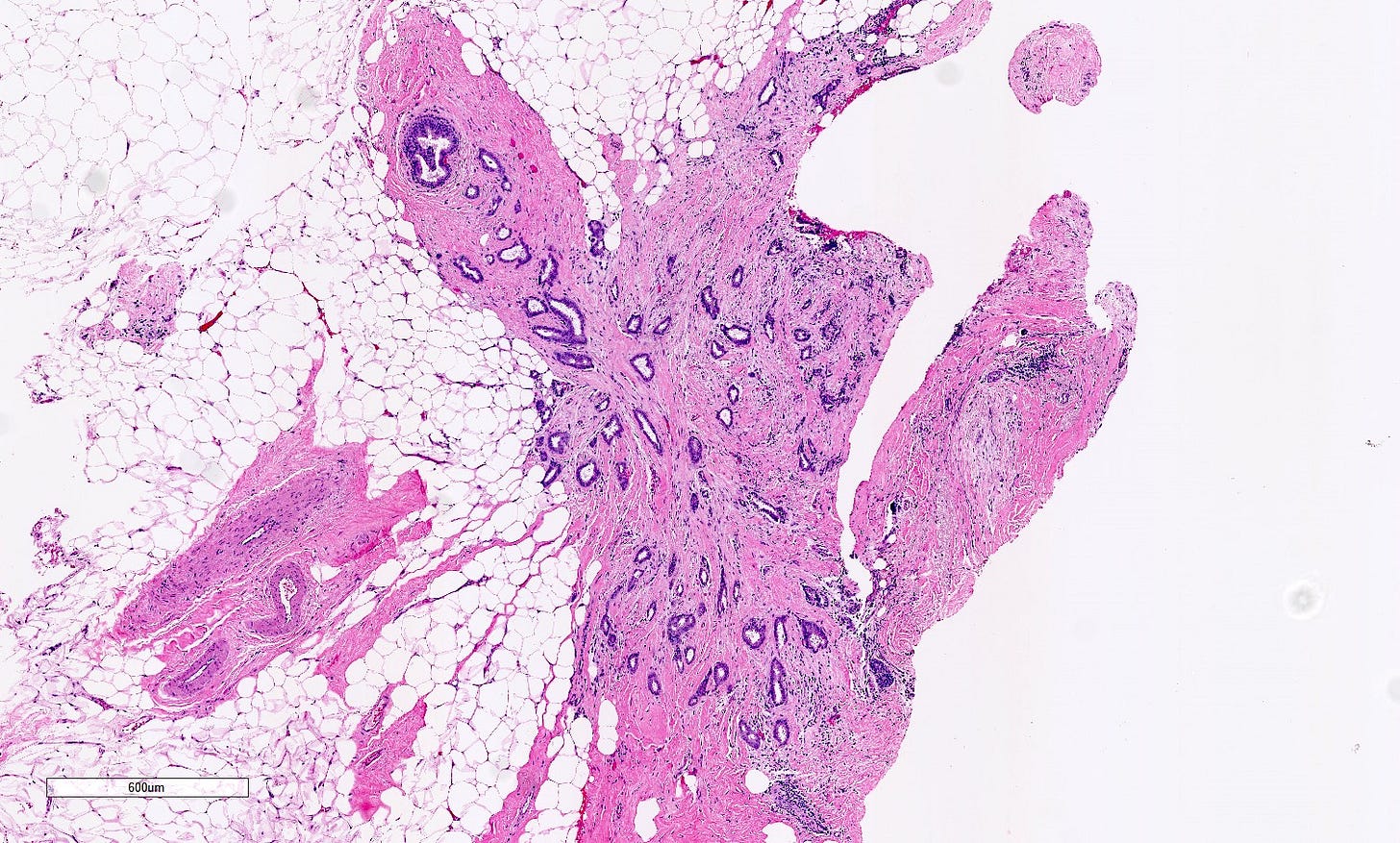
Precursor of triple negative carcinoma - microglandular adenosis
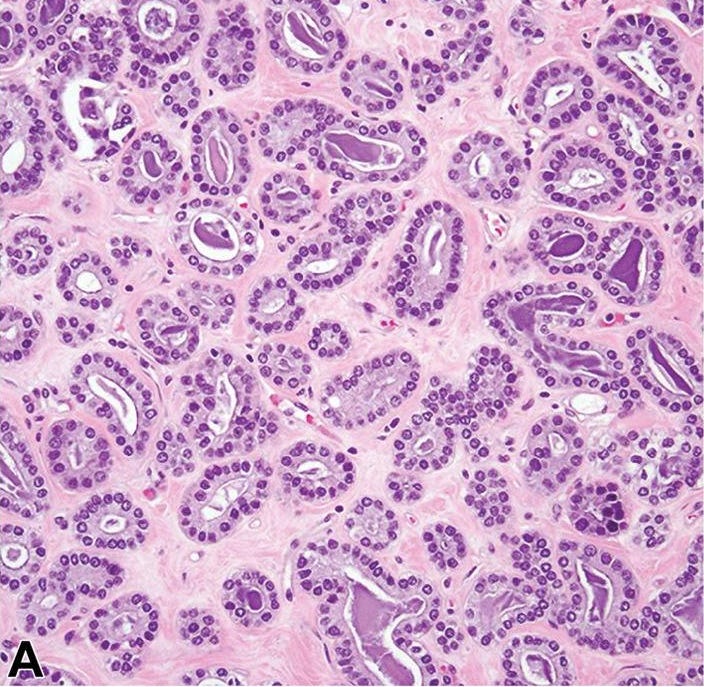
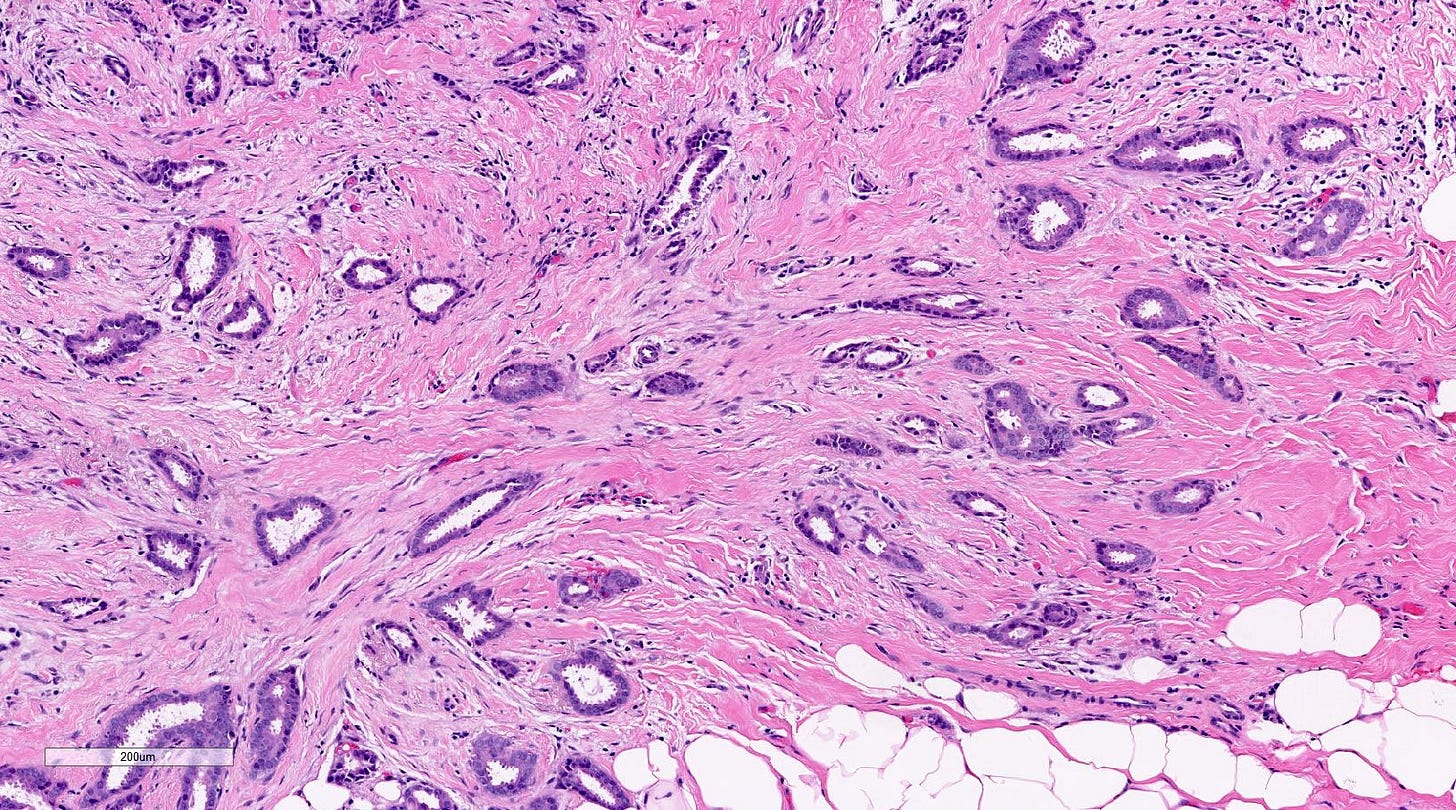
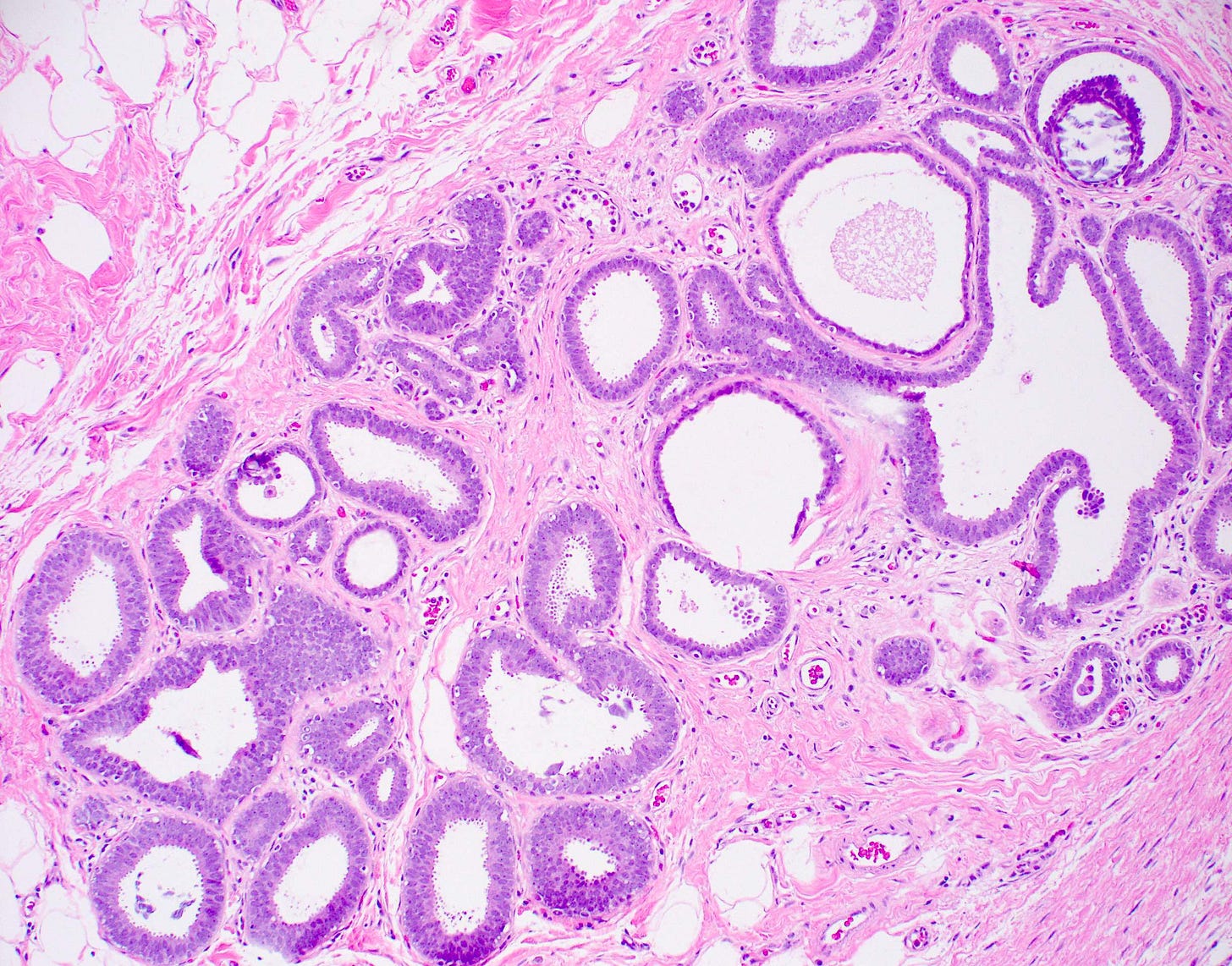
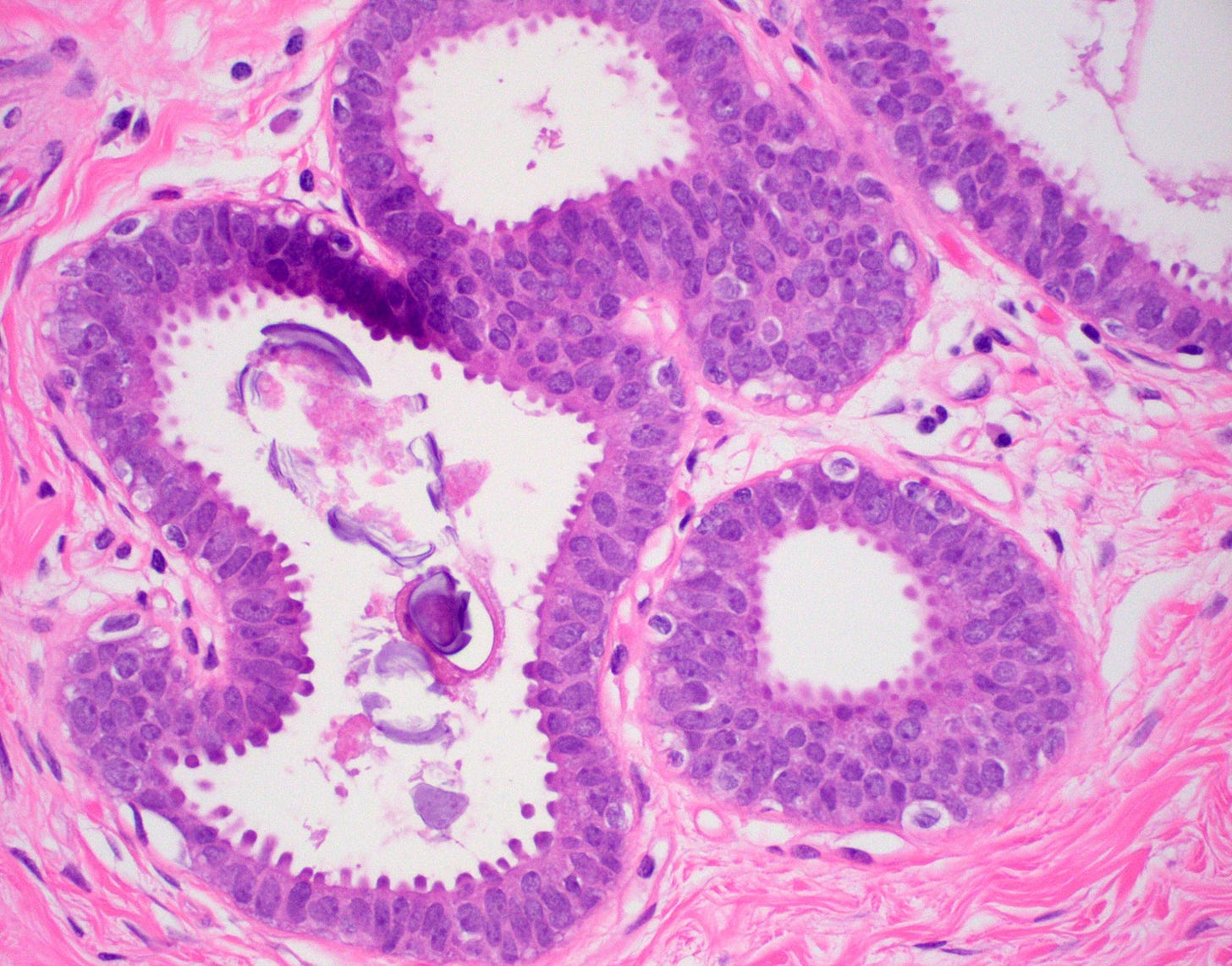
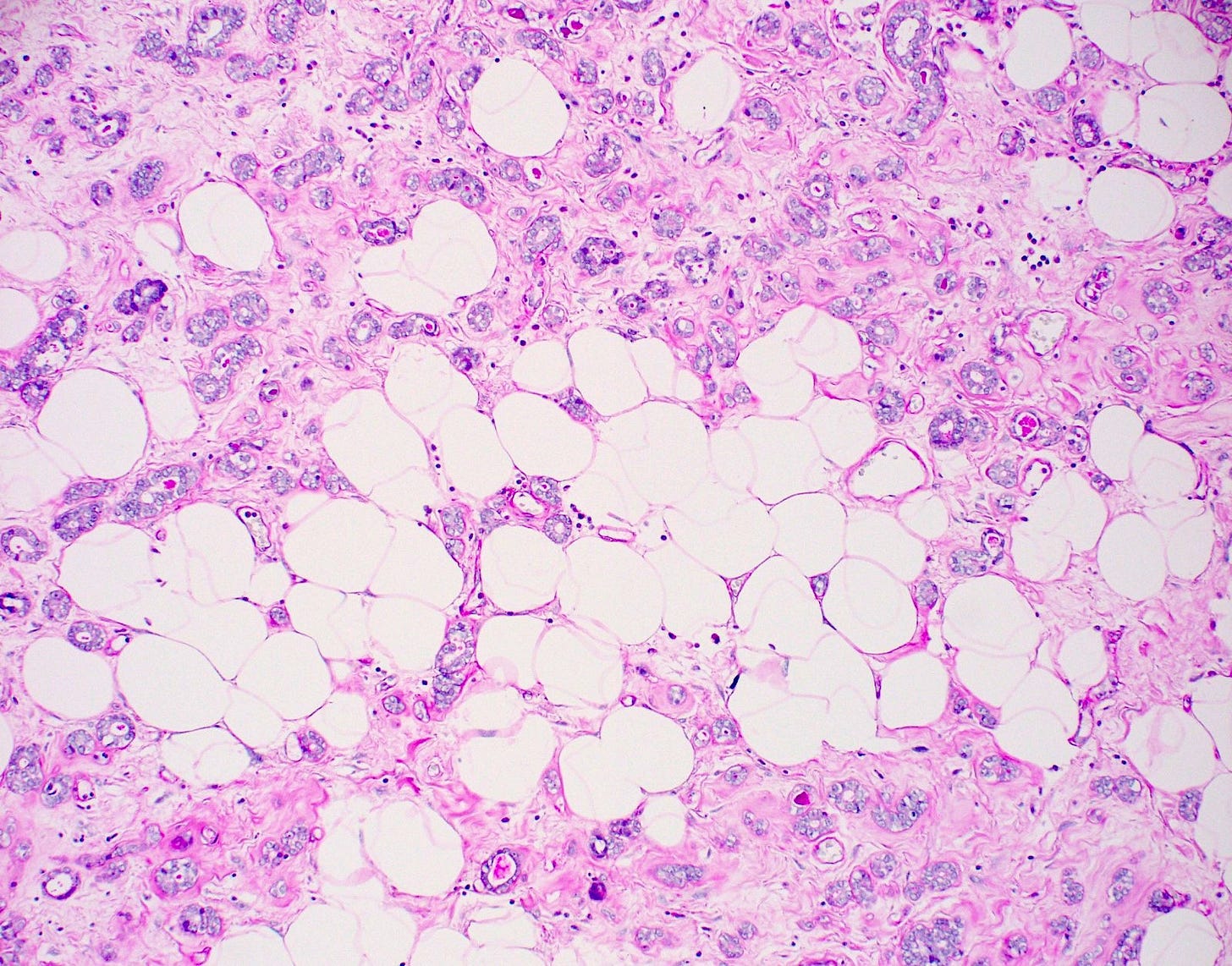
Microglandular adenosis is a precursor for some subtypes of triple negative carcinoma, but not for BRCA-associated carcinoma, which will be discussed in the next essay. Microglandular adenosis is a rare, benign lesion composed of a haphazard, irregularly distributed collection of small, bland, rounded, open glands with eosinophilic secretions. There are usually no apocrine snouts, no nucleoli, no mitotic figures and no necrosis. It may mimic invasive carcinoma due to its infiltrative growth into fibrous and adipose tissue without a stromal response. In addition, it has a single epithelial layer which contains a basement membrane but there is no myoepithelial layer. It is positive for cytokeratins and S100 and negative for ER, PR and HER2 immunohistochemical stains.
An atypical microscopic variant of microglandular adenosis shows increased complexity including multilayered epithelium, fused glandular units, luminal bridging and cribriform architecture. There is also mild cytologic atypia, hyperchromatic nuclei, prominent nucleoli and occasional mitotic figures.
Microglandular adenosis appears to constitute a heterogeneous group of lesions. Some cases with classic and atypical microglandular adenosis are clonal and considered precursors of triple negative carcinoma of the breast, which has similar immunohistochemical features; specimens with both microglandular adenosis (classic or atypical) and triple negative carcinoma may have identical mutation patterns. The microglandular adenosis component may have TP53 mutations and high levels of genomic instability which cause disruptions in signaling and protein networks leading to malignant progression. Cases of microglandular adenosis without atypia or any other precursor lesions may have little risk of malignant progression.
Grossly, microglandular adenosis is typically indiscernible from surrounding tissue. If a mass is present, it is usually associated with a coexisting carcinoma.
Precursor of triple negative carcinoma - microglandular adenosis - microscopic images
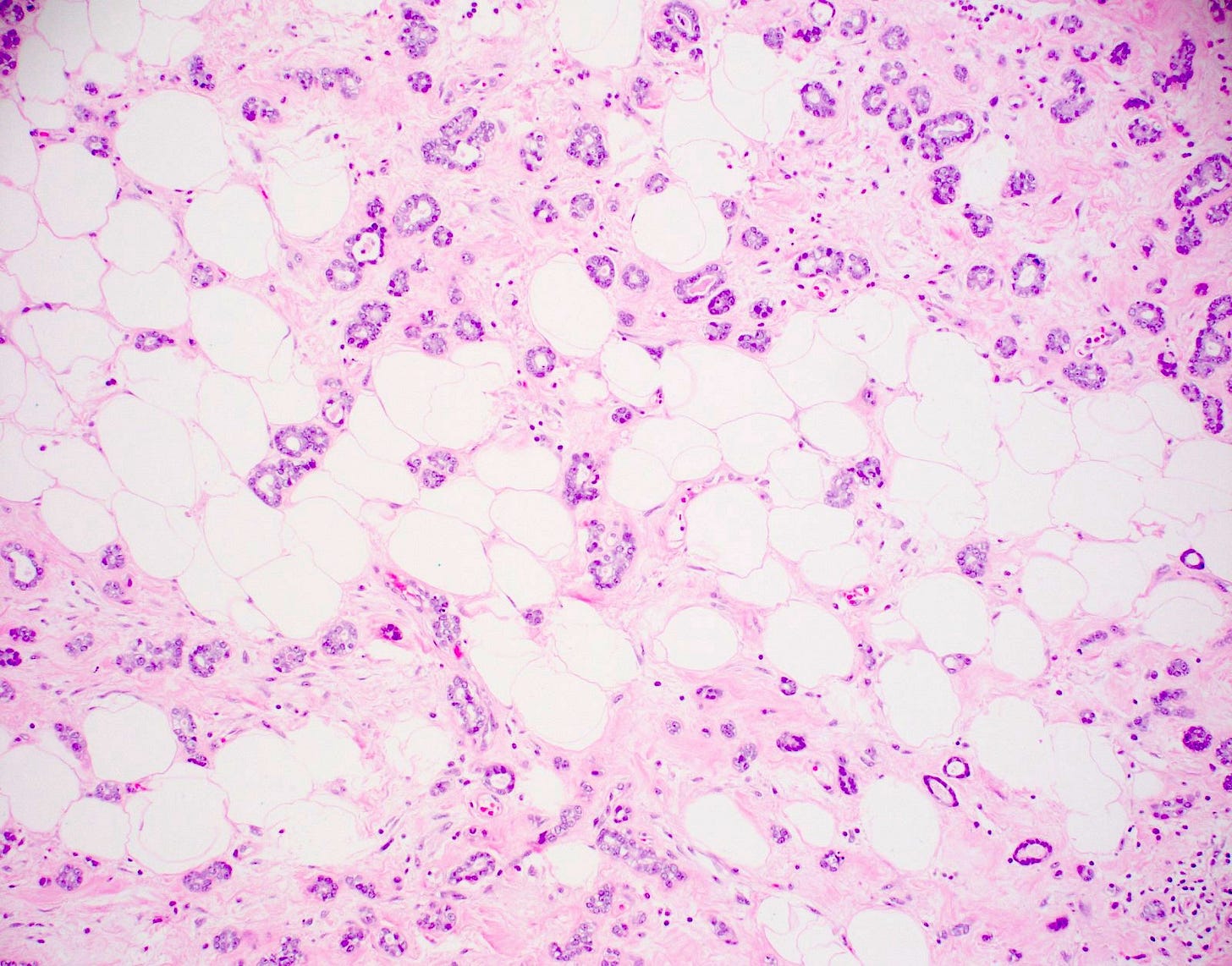
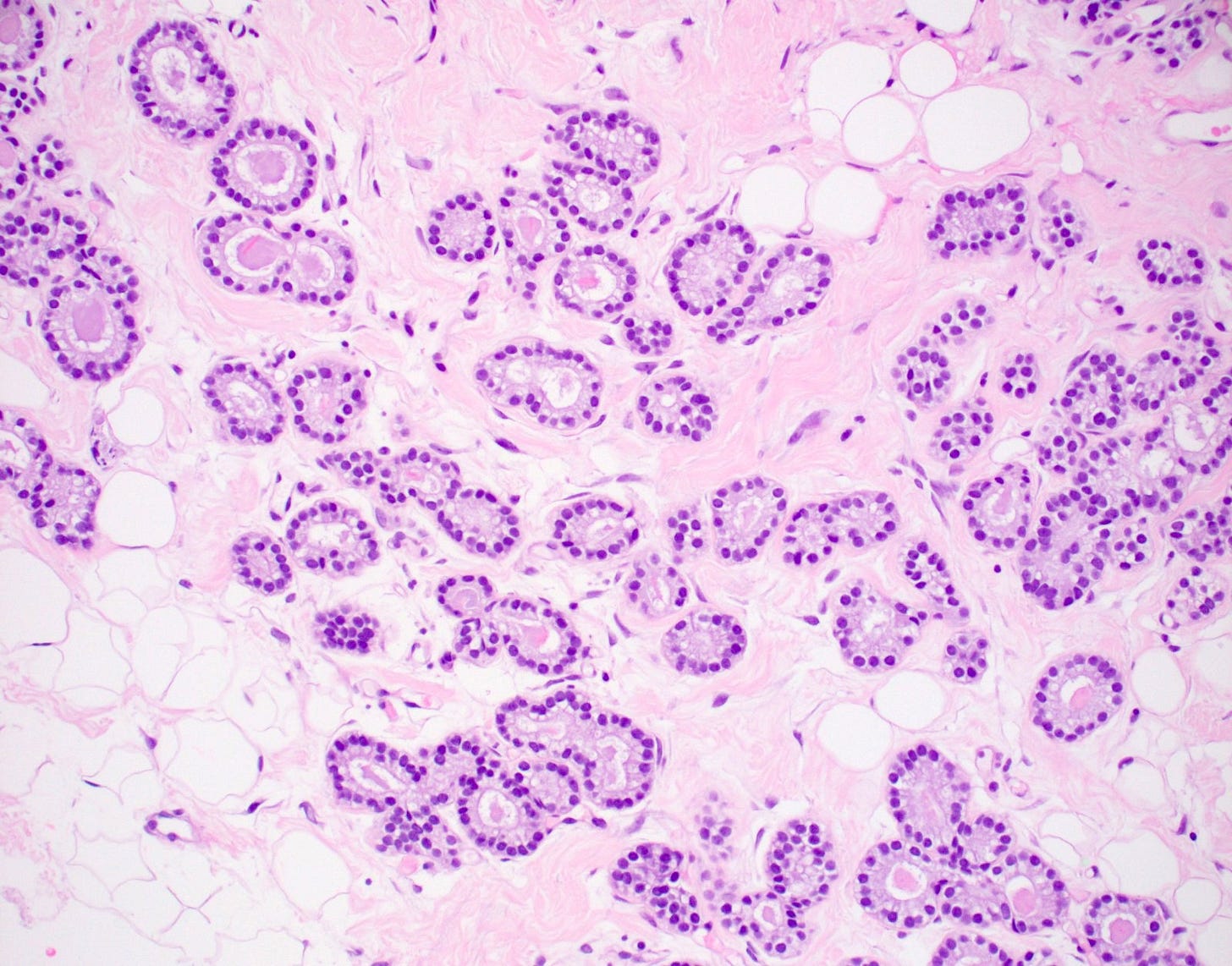

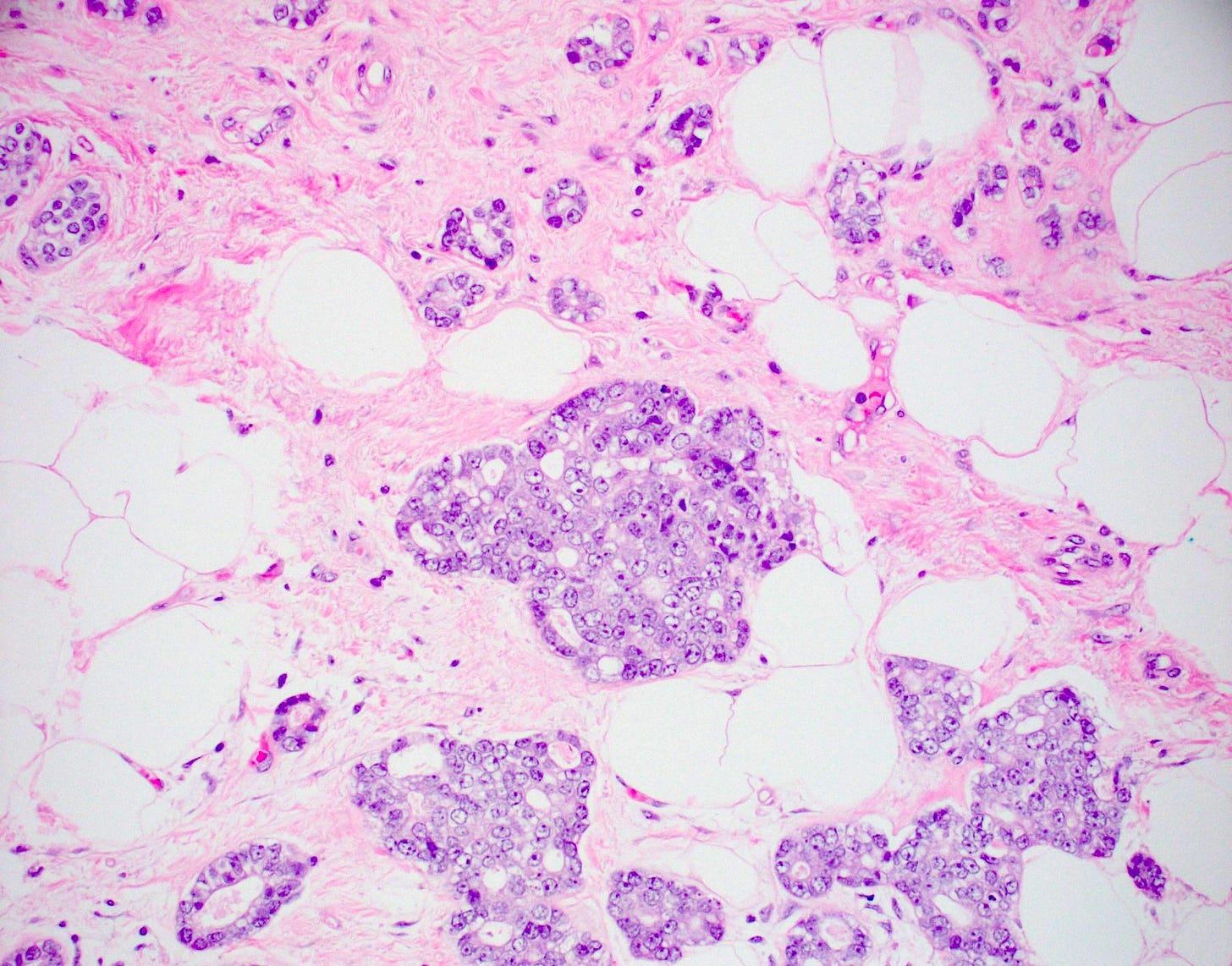
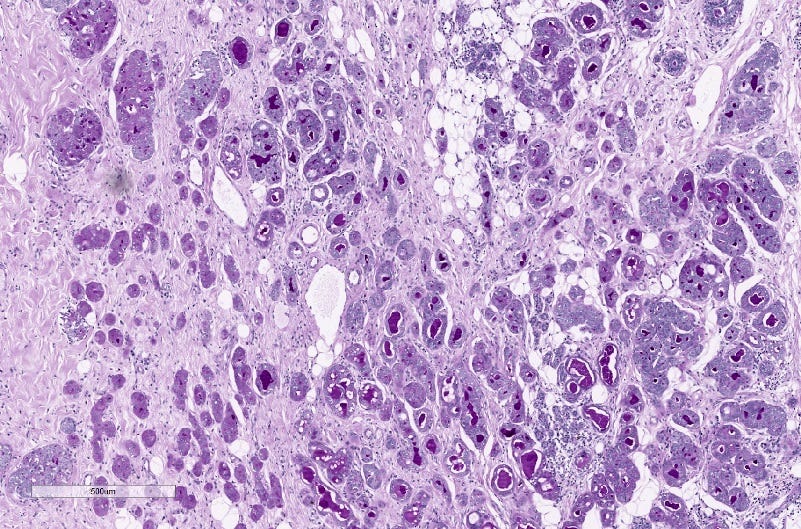
Acinic cell carcinoma of the breast
Acinic cell carcinoma (also called acinar cell carcinoma) is a common salivary gland malignancy (10 - 17% of primary salivary gland malignancies). It also occurs in the pancreas (1 - 2% of adult pancreatic neoplasms [benign and malignant], 15% of neoplasms in children) but occurs only rarely in the breast (< 100 cases reported).
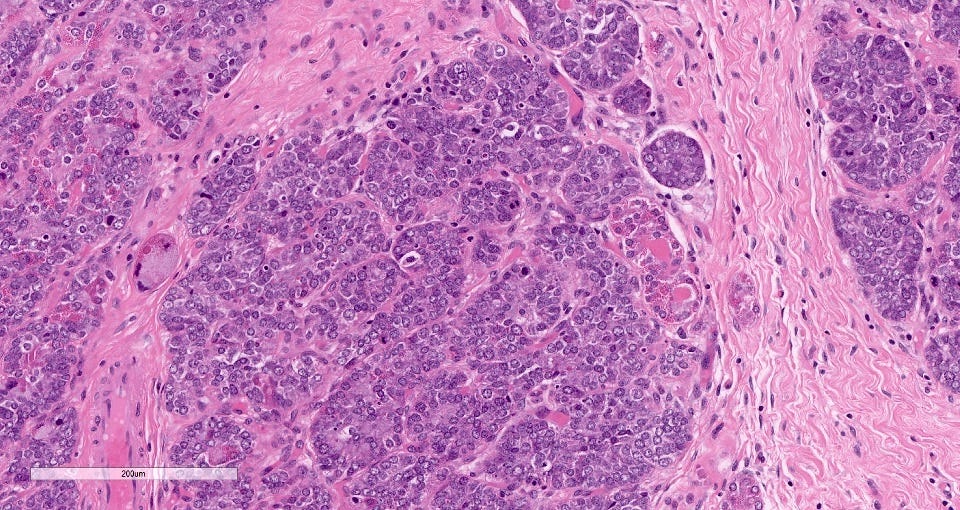
Acinic cell carcinoma is characterized by infiltrative microglandular and solid growth patterns with serous acinar cell differentiation (cytoplasmic zymogen granules that stain positive for periodic acid-Schiff with diastase) in some neoplastic cells. It has a triple negative phenotype (ER negative, PR negative, HER2 negative) but is less aggressive than traditional triple negative breast carcinomas. Its molecular features resemble triple negative breast cancers more than its salivary gland counterpart and lack the characteristic NR4A3 rearrangement or overexpression typically found it its salivary gland counterpart.
Acinic cell carcinoma has axillary nodal metastases in fewer than 20% of cases. Distant metastases occur in a similar pattern as invasive ductal carcinoma of no special type and peritoneal metastases may rarely develop.
Treatment consists of breast conserving surgery or mastectomy, typically with sentinel lymph node excision or axillary lymph node dissection. The use of chemotherapy, radiotherapy and hormonal therapy is variable.
Grossly, the tumors are poorly defined, gray-brown and rubbery. The microscopic features were described above.
The precursor for acinic cell carcinoma is also microglandular adenosis and both have similar molecular features. In fact, Paul Rosen believes that acinic cell carcinoma is simply an invasive carcinoma with acinic cell differentiation arising in microglandular adenosis, which explains why it lacks the molecular features of salivary gland acinic cell carcinoma. Normal breast glands may also show acinar differentiation so this type of differentiation is not unexpected.
Acinic cell carcinoma of the breast - microscopic images
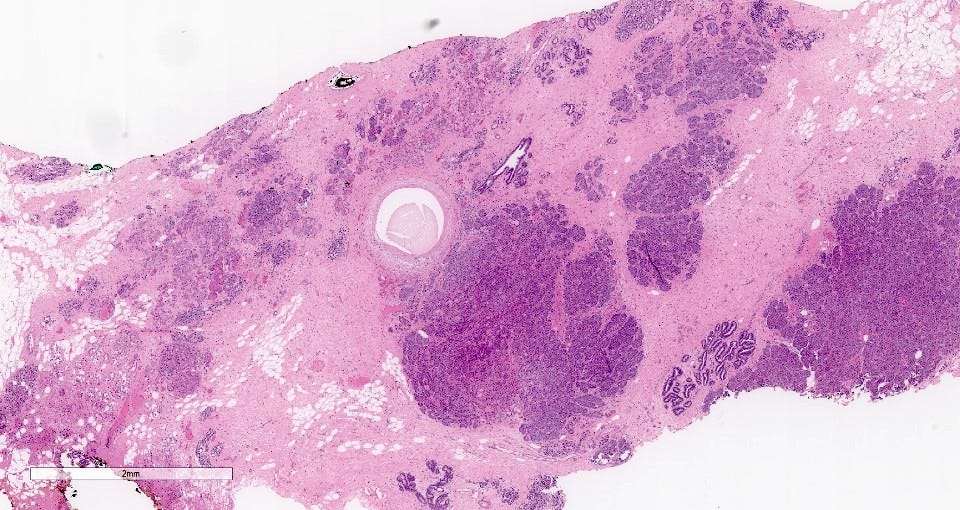
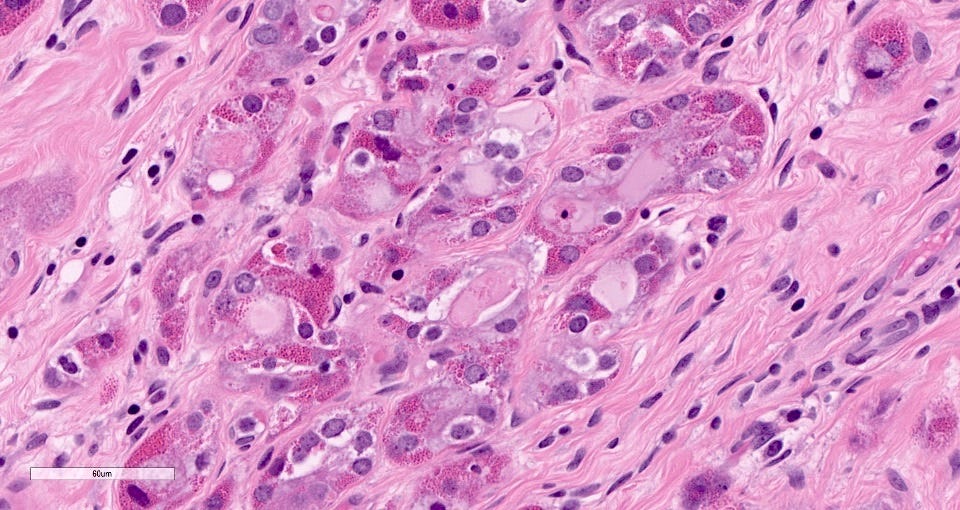
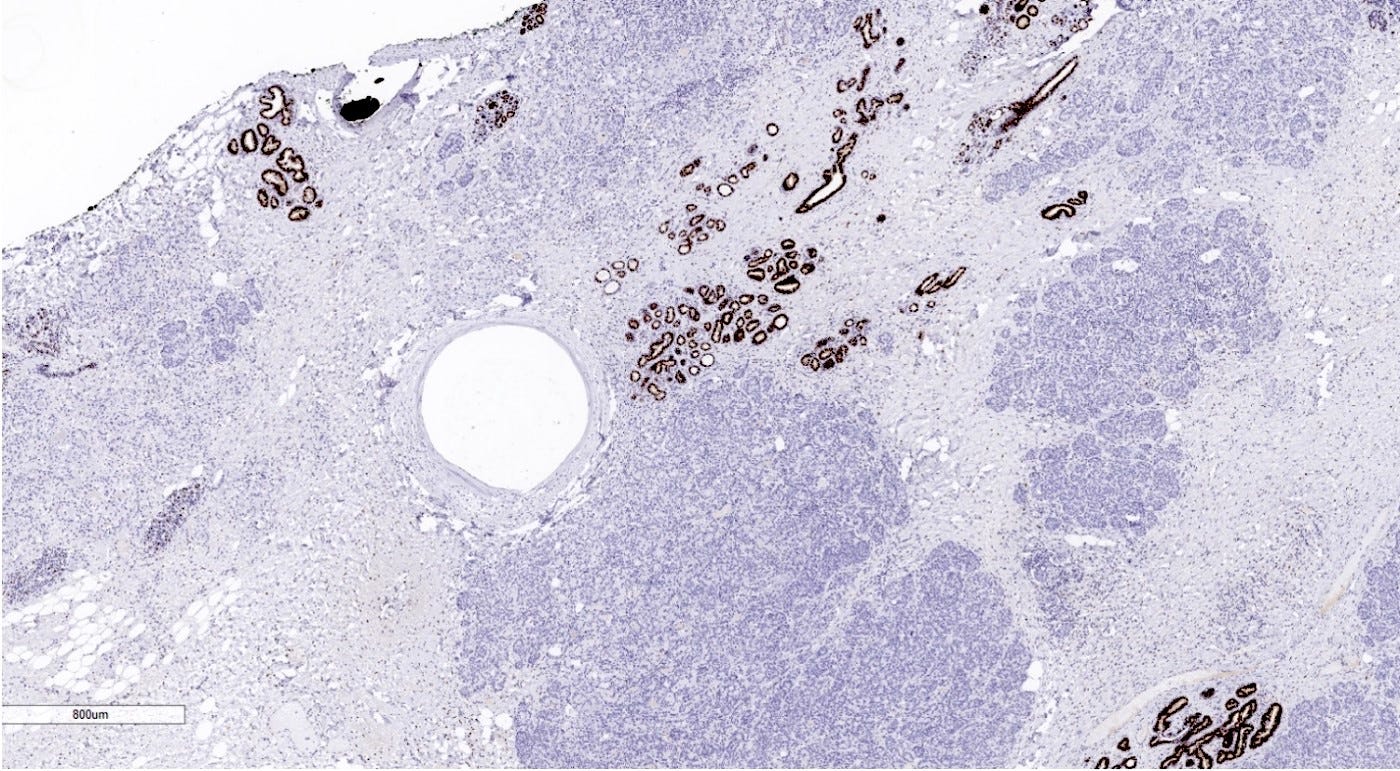
Precursor of acinic cell carcinoma - microglandular adenosis - microscopic images
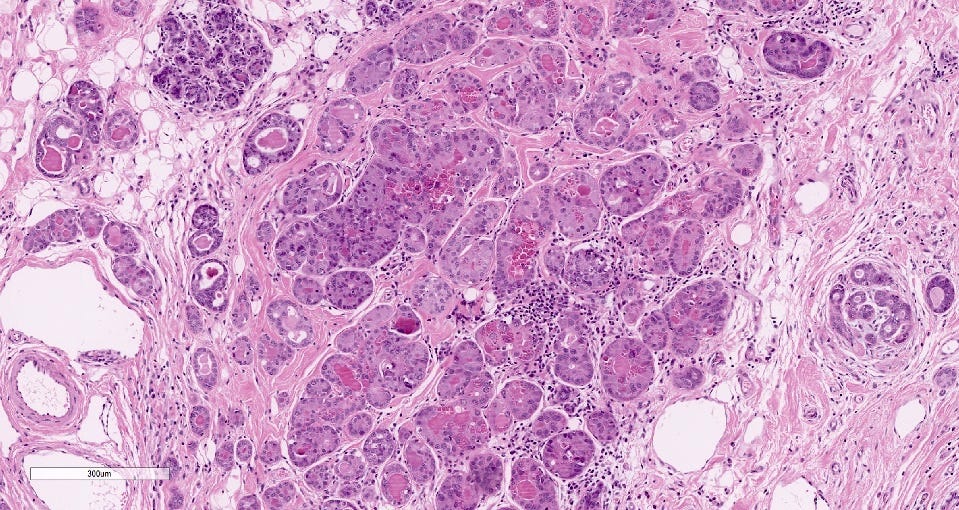
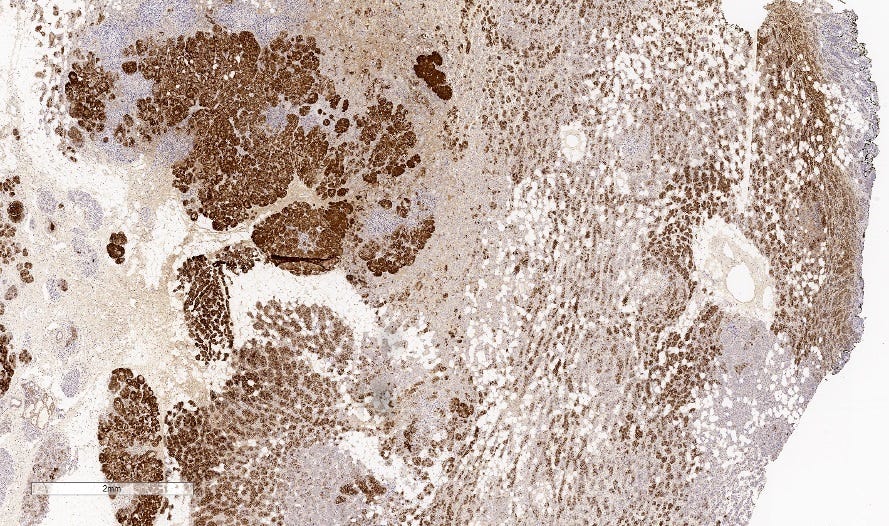
Metaplastic carcinoma of the breast
Metaplastic carcinoma is an uncommon (0.2 to 1.0% of all invasive breast cancers) type of triple negative breast carcinoma characterized by differentiation toward squamous cells or mesenchymal looking elements. In cases lacking components of ductal carcinoma in situ or conventional breast carcinoma, immunohistochemical stains are needed to confirm its epithelial differentiation.
Clinically, it presents similar to invasive breast carcinoma of no special type. The tumors tend to be larger, with higher stage but less nodal involvement. Treatment consists of mastectomy or local excision, with or without radiation and chemotherapy. In general, there is a poor response to neoadjuvant systemic therapy
Metaplastic carcinoma has 3 subtypes: fibromatosis-like, low grade adenosquamous and squamous cell carcinoma. The first 2 are associated with less aggressive behavior, as are matrix producing metaplastic carcinomas. High grade spindle cell carcinomas, squamous cell carcinomas, high grade adenosquamous carcinomas and some mixed metaplastic carcinomas are associated with a worse prognosis.
Grossly, the tumors average 4 cm (range, 2 to 10+ cm) and are often firm, well circumscribed and solid. They have a pearly white to grayish and glistening cut surface in areas of squamous and chondroid differentiation
Microscopically, these tumors are heterogeneous. They are either pure carcinomas (low grade adenosquamous carcinoma, high grade adenosquamous carcinoma, squamous cell carcinoma), monophasic sarcomatoid carcinomas (fibromatosis-like metaplastic carcinoma, spindle cell carcinoma) or biphasic epithelial and sarcomatoid carcinomas.
They may have heterologous mesenchymal components with combinations of chondroid, osseous, rhabdomyosarcomatous, angiosarcomatous, liposarcomatous and neuroglial features. Atypia varies from minimal to frankly malignant.
Pathologists should look carefully to identify epithelial components, using immunohistochemistry if necessary.
Metaplastic squamous cell carcinomas may have squamous metaplasia as a precursor.
Metaplastic squamous cell carcinoma of the breast - gross and microscopic images

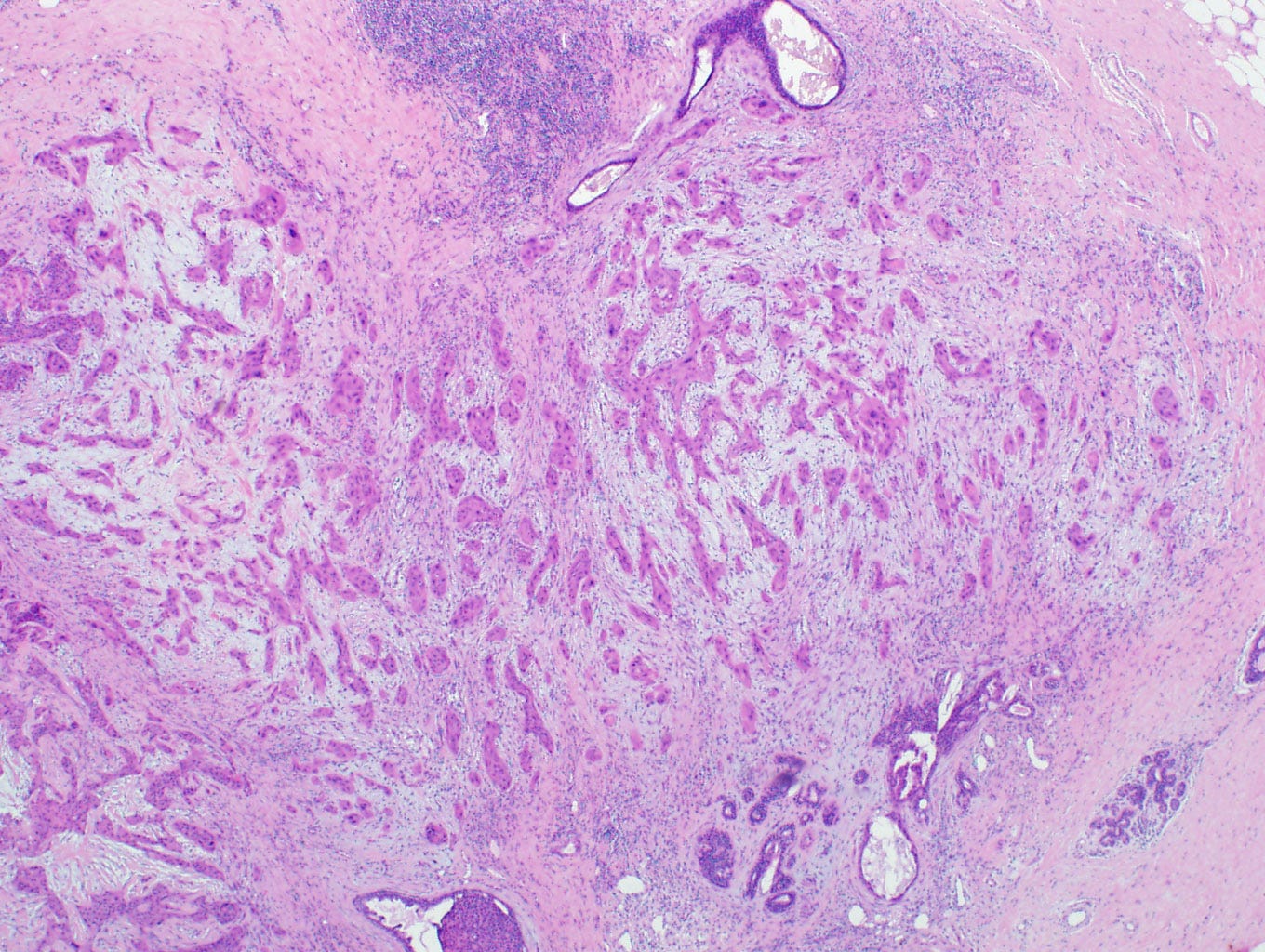
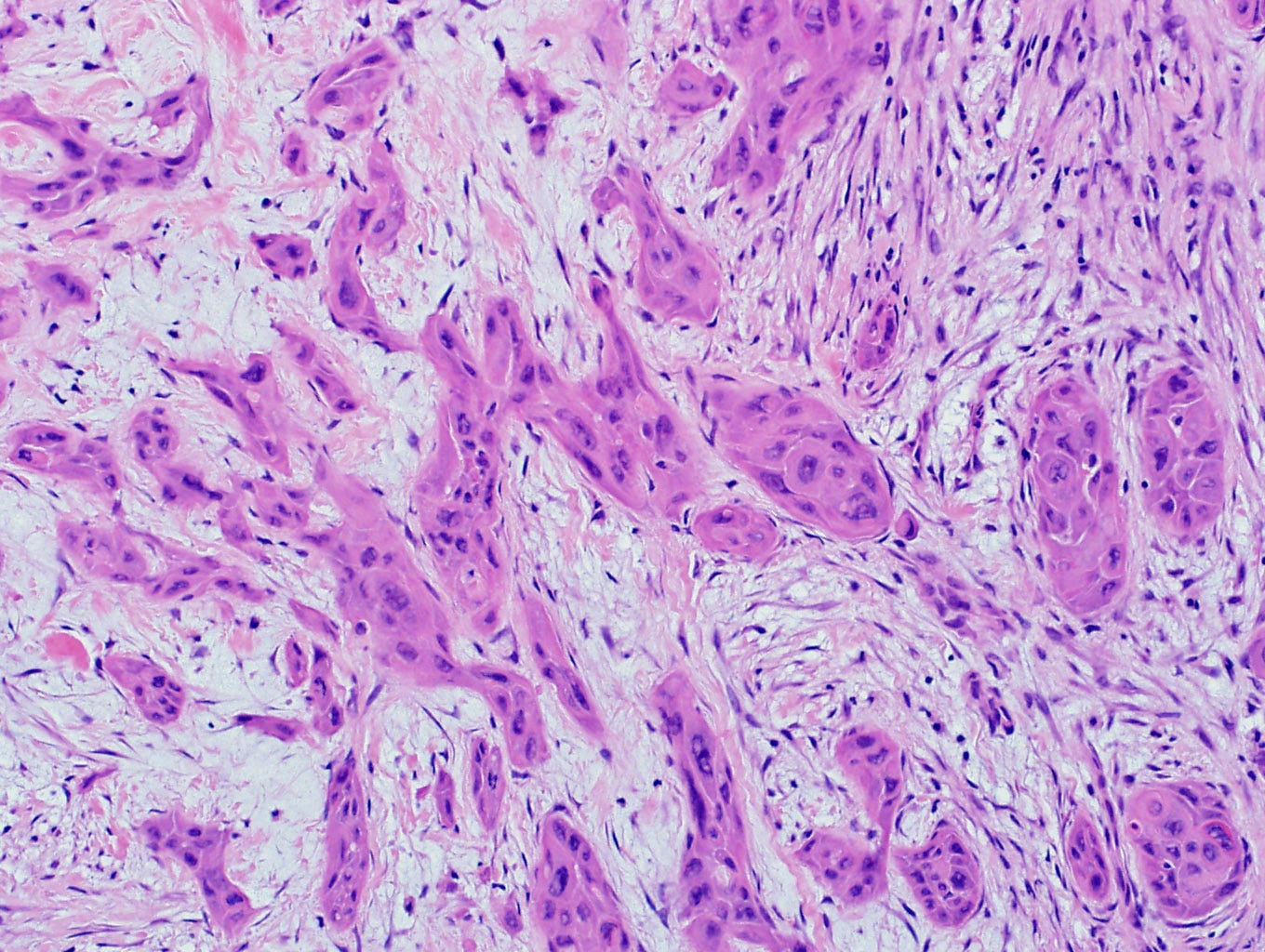
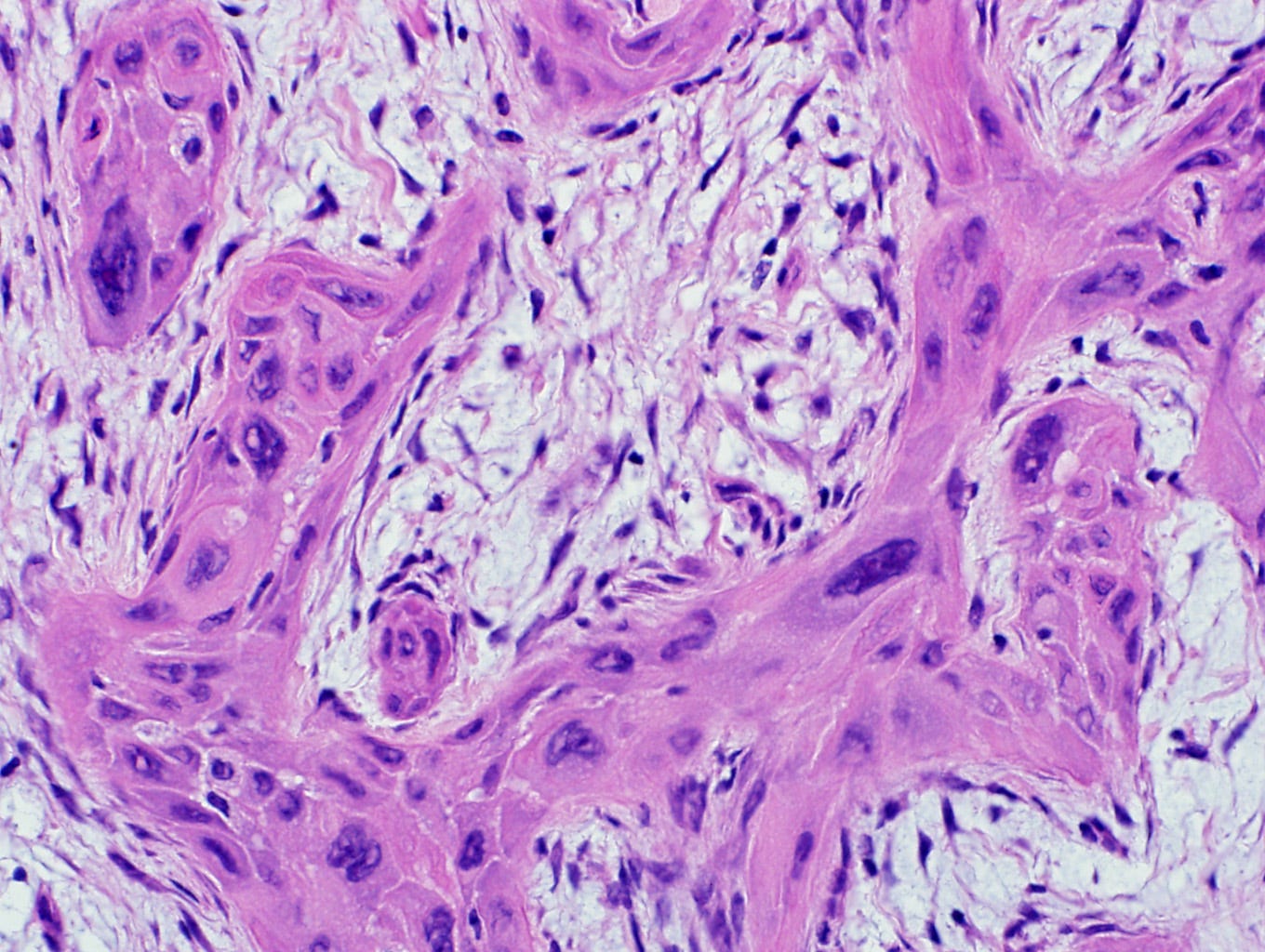
Precursor of metaplastic squamous cell carcinoma - squamous cell metaplasia
Squamous metaplasia of the breast ductal epithelium is an uncommon microscopic finding. It is associated with fibroadenomas, phyllodes tumors, cysts, abscesses, chronic inflammation, ductal or lobular hyperplasia and papillomas. It arises from the remodeling of breast ductal epithelium toward a stratified epidermoid squamous epithelium comprising multiple layers of cells, that may be more resistant to injury.
DNA damage promotes squamous cell metaplasia in both the lung and breast through mitotic checkpoints. However, the rarity of both squamous metaplasia and squamous cell carcinoma in the breast compared to the lung suggests that environmental factors are an important factor (i.e. direct exposure to cigarette smoke is much higher in the lung than breast).
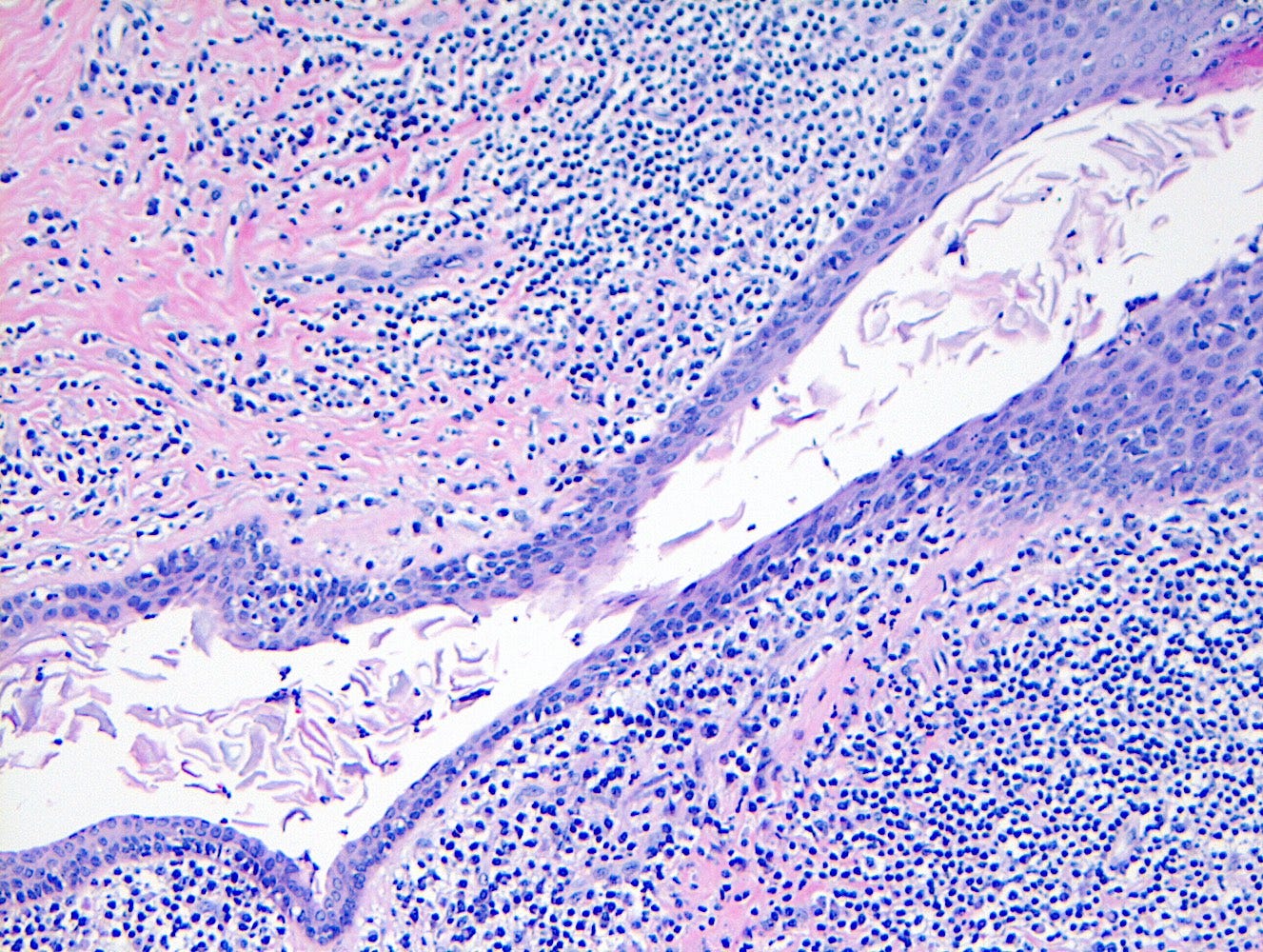
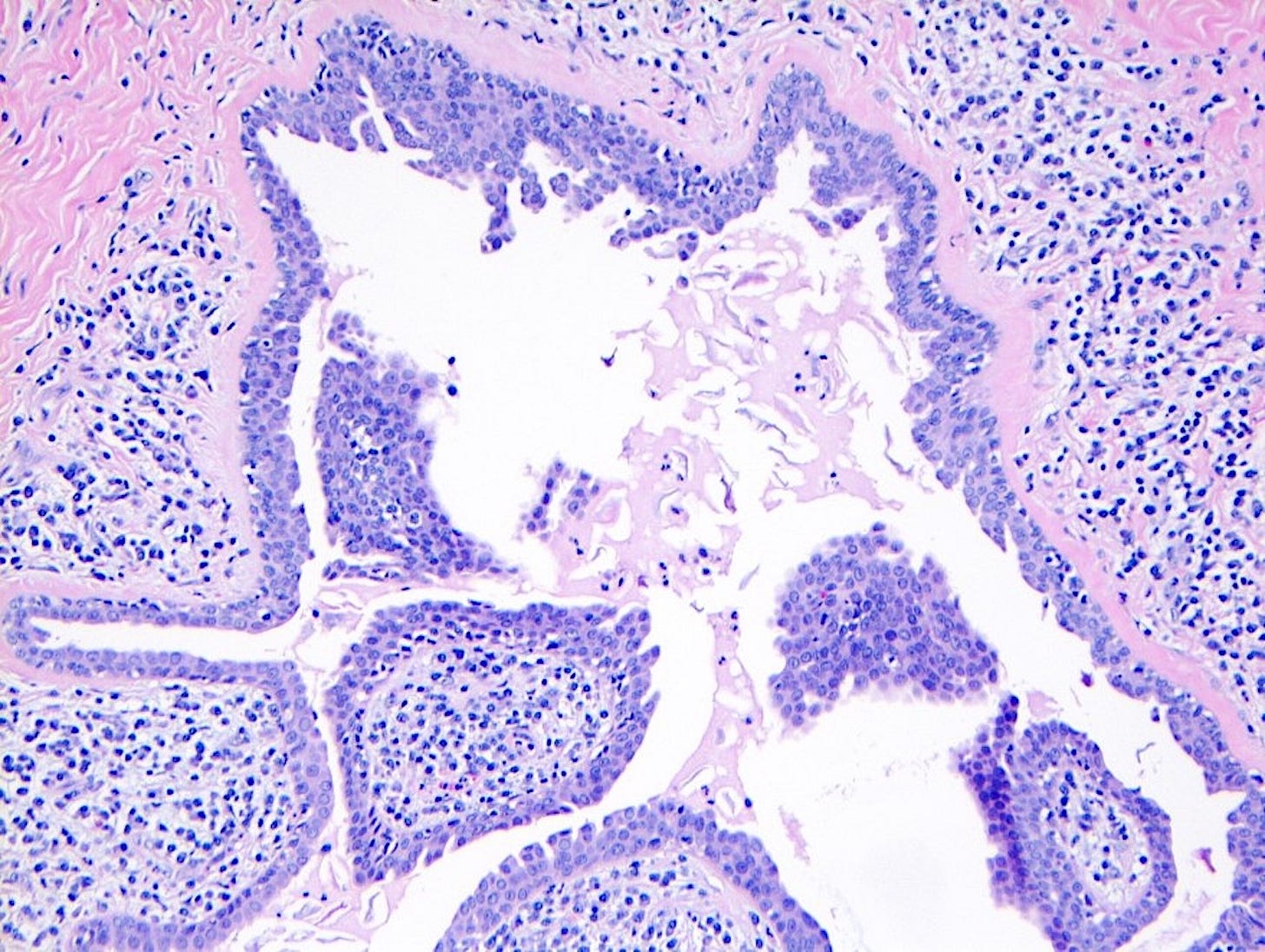
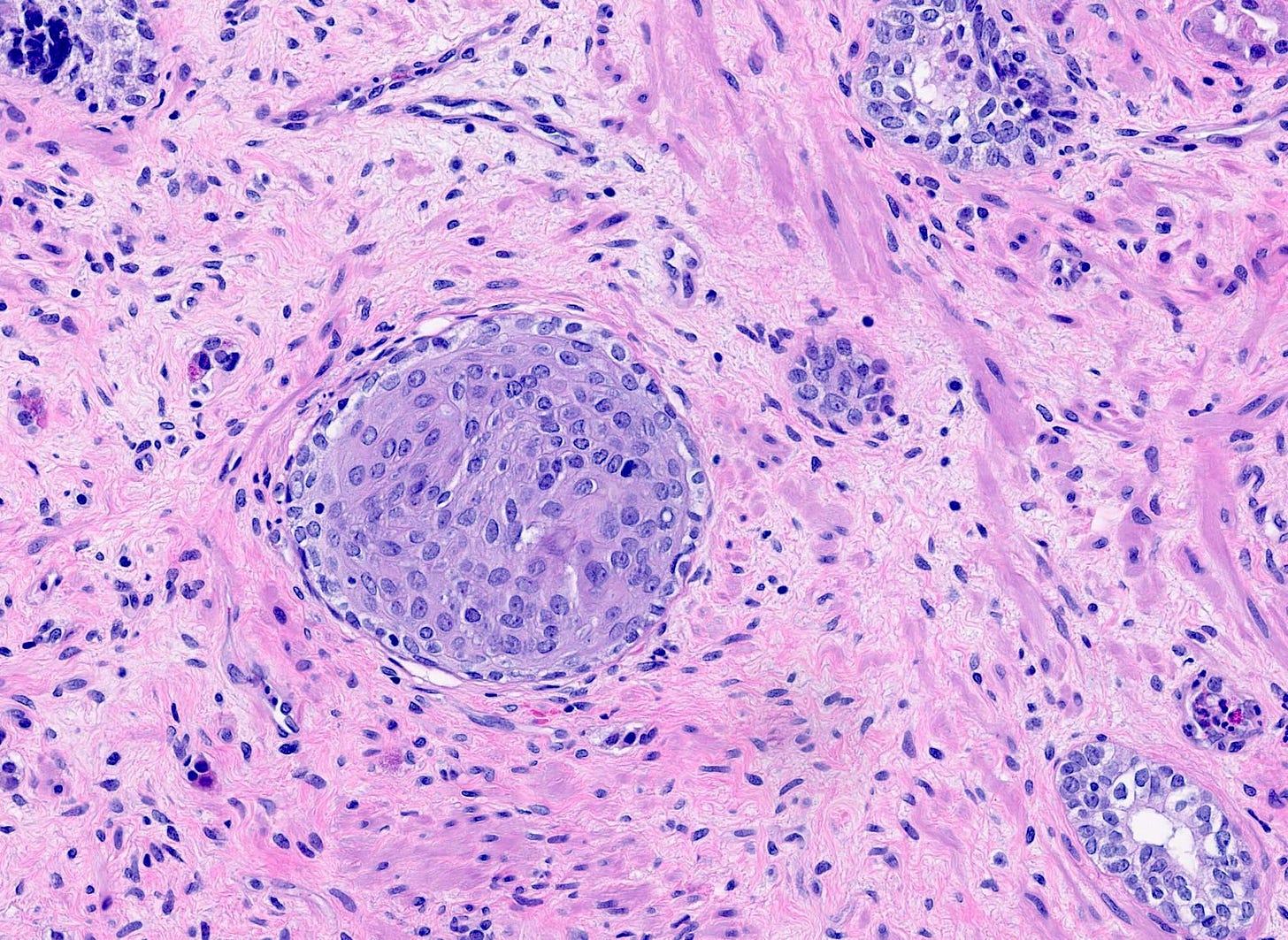
Precursor of metaplastic squamous cell carcinoma - squamous cell metaplasia - microscopic images
Due to its rarity, I have no images of squamous cell metaplasia associated with metaplastic squamous cell carcinoma of the breast. I have included images that highlight its microscopic features from SMOLD, an inflammatory condition of breast ducts and treated prostate cancer.
The next essay, part 6d1, will discuss three papillary breast malignancies with known precursors.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me at https://www.linkedin.com/in/nat-pernick-8967765/ (LinkedIn), npernickmich (Threads and Instagram), natpernick.bsky.social (Bluesky) or @nat385440b (Tribel).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week we post interesting cancer related images of malignancies with diagnoses plus articles of interest. Please also read our CCN essays.
Latest versions of our cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email me at Nat@PathologyOutlines.com - Unfortunately, I cannot provide medical advice.
I also publish Notes at https://substack.com/note. Subscribers will automatically see my Notes.