Cancer precursor project - breast cancer, part 6e
6 January 2025, revised 15 August 2025
This weekly medical blog focuses on science, not politics. For political commentary, please see my separate daily blog. Feel free to skip any essays or sections that are not of interest - no offense taken.
This post is part of my cancer precursor project, which explores how cancer arises. Part 6 addresses breast cancer. Click here for links to essays on all 46 types of breast cancer.
In part 6a of our cancer precursor project, we discussed breast cancer mortality, breast cancer risk factors and how breast cancer arises. In part 6b, we discussed breast anatomy and histology and started our discussion of the eleven invasive breast cancers with known precursors, which continued in part 6c, part 6d and part 6d1.
To date, the known precursors of invasive breast cancer are:
DCIS (ductal carcinoma in situ) - low grade and high grade
Encapsulated papillary carcinoma (noninvasive)
Intraductal papillary carcinoma (noninvasive)
LCIS - lobular carcinoma in situ - classic, florid and pleomorphic
MGA (microglandular adenosis) - classic and atypical
Solid papillary carcinoma (noninvasive)
Squamous metaplasia
The 14 breast cancers with known precursors are:
We now discuss breast cancers without known precursors beginning with BRCA associated carcinoma, which lacks the precursor of microglandular adenosis seen in other triple negative breast cancers.
BRCA associated breast carcinoma is caused by a germline mutation in the BRCA1 (usually) or BRCA2 genes, which encode for BRCA1 / 2 proteins. These proteins play crucial roles in the repair of double stranded DNA breaks. They also have roles in cell cycle regulation, chromatin remodeling and transcription control. Most BRCA1 / 2 mutations lead to nonfunctional BRCA1 or BRCA2 proteins, which limit the cell’s ability to repair DNA damage and increase genomic instability, thus leading to a higher rate of new mutations. As a result, more than 60% of women with these mutations develop breast cancer in their lifetime, compared to 13% in the general population (it also causes other cancers).
BRCA associated carcinoma typically occurs at a younger age (< 50 years) and often involves both breasts. Microscopically, most BRCA1 associated breast cancers are high grade (poorly differentiated) and well demarcated with pushing margins and medullary-like features. BRCA2 associated breast cancers are more heterogeneous in terms of grade and subtype, with a larger proportion being low and intermediate grade compared to BRCA1 associated cancers.
There are three major physiologic processes to prevent malignancy from DNA changes, all of which are disrupted in BRCA associated carcinoma:
1. BRCA proteins repair DNA double strand breaks, which are inevitable in life and may cause malignant transformation of cells (they can also cause cell death or dysfunctional cells). Of note, DNA double strand breaks are deliberately formed during meiosis (production of egg and sperm cells), T cell receptor formation and B cell immunoglobulin class switching (part of the immune system). DNA double strand breaks may be associated with cancer when due to ionizing radiation, chemical exposure or physiologic DNA replication and repair. To keep DNA intact, our genome has 169 DNA repair genes and two major repair mechanisms (homologous recombination and non-homologous end joining). Homologous recombination uses BRCA1/2 proteins, but it is ineffective in patients with BRCA mutations.
2. DNA damaged cells that are not repaired by BRCA1/2 proteins (or otherwise) may undergo apoptosis (programmed cell death), mediated by p53, to prevent them from becoming malignant.
3. DNA damaged cells that persist are typically destroyed by NK cells or CD8+ T cells in the immune system.
BRCA1/2 mutations may cause these three processes to be ineffective because:
The inactive BRCA1/2 proteins cannot repair DNA damage, leading to a large number of DNA mutations.
The inactive BRCA1/2 proteins may also promote genetic instability, leading to an increasing rate of new mutations.
The inactive BRCA1/2 proteins may damage p53 or other components of the apoptosis pathway, which prevents apoptosis from destroying the potentially malignant cells and also promotes genomic instability.
The large number of cells with DNA damage may exhaust or overwhelm the immune system so it cannot function properly.
BRCA associated carcinoma may lack a premalignant precursor because:
Its malignant transformation may be characterized initially by the slow accumulation of mutations that are not associated with microscopic changes in the initial cell or its descendants. As the number of mutations increases, a tipping point or critical mass of mutated proteins occurs, leading to an avalanche of rapid malignant changes with no precursors evident, particularly in BRCA1 related cancers.
The nature of the tipping point may be based on mutations that facilitate the expression of gene networks key to unicellular behaviors, based on the atavism theory of cancer.
The large set of mutations that arise in BRCA1 associated carcinomas may include those negating the properties of epithelial cell junctions and basement membranes (or the myoepithelial cells adjacent to basement membranes) which typically slow down the malignant process and produce precursor lesions.
BRCA associated breast carcinoma - microscopic images (these are due to BRCA1 mutations)
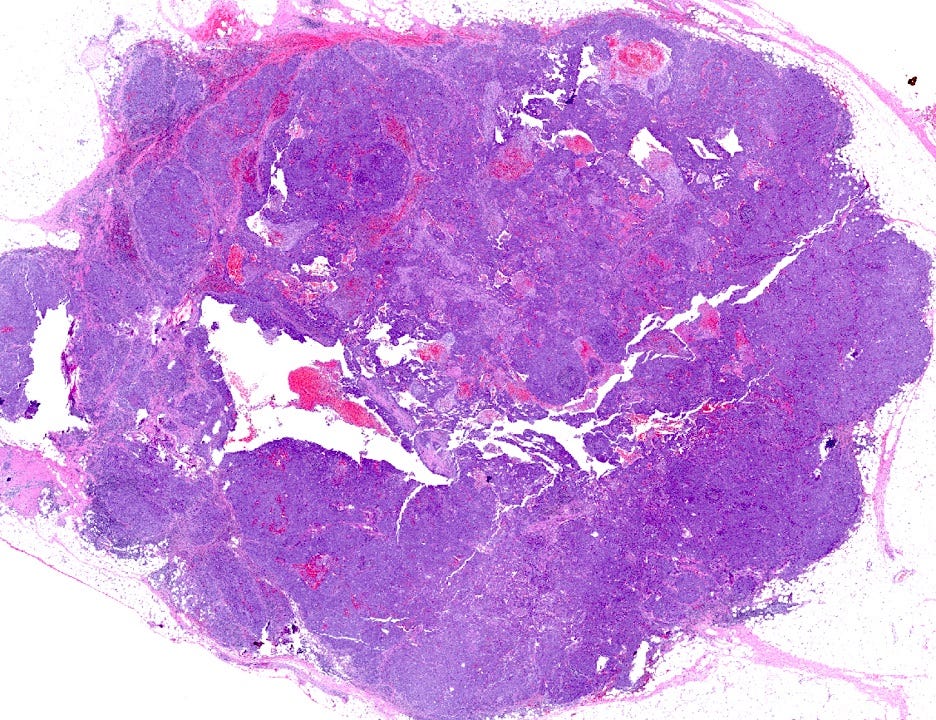
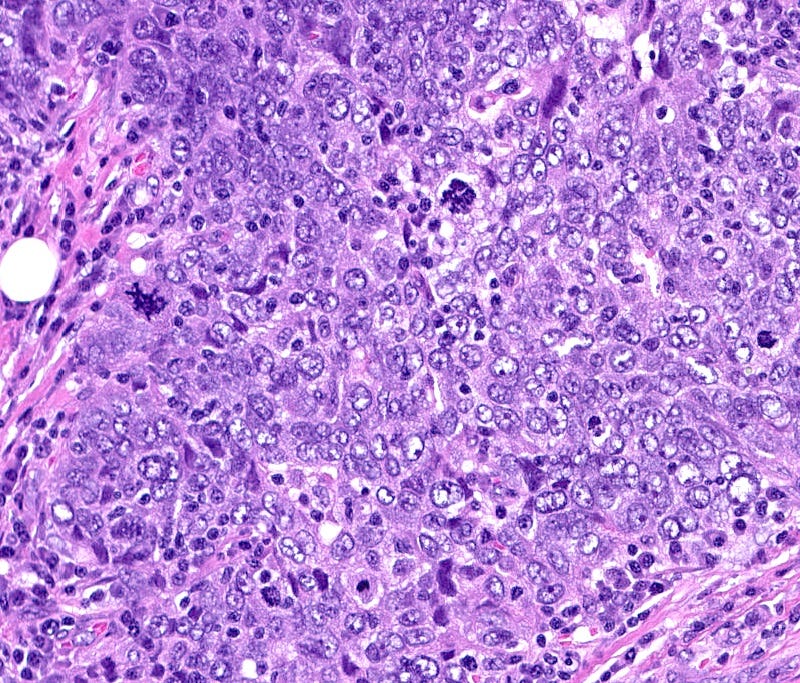
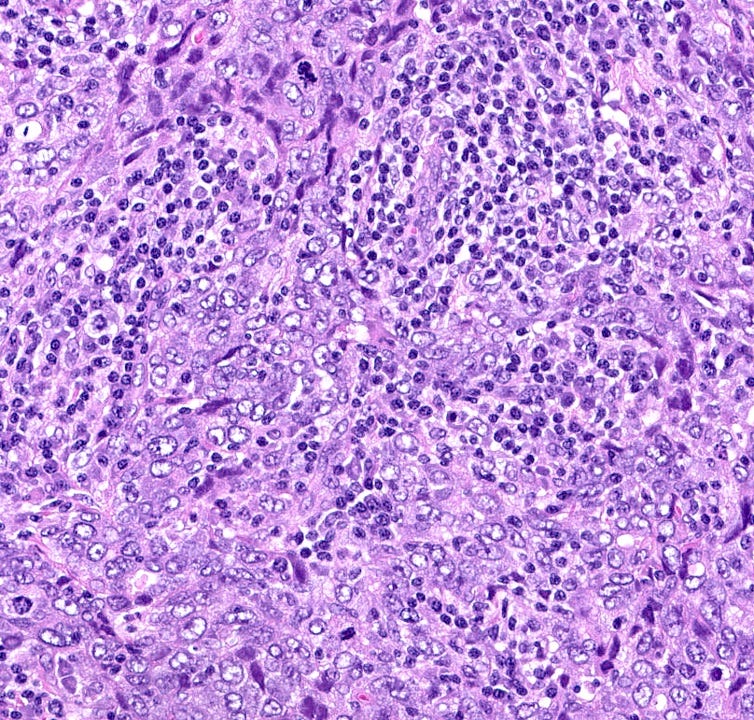

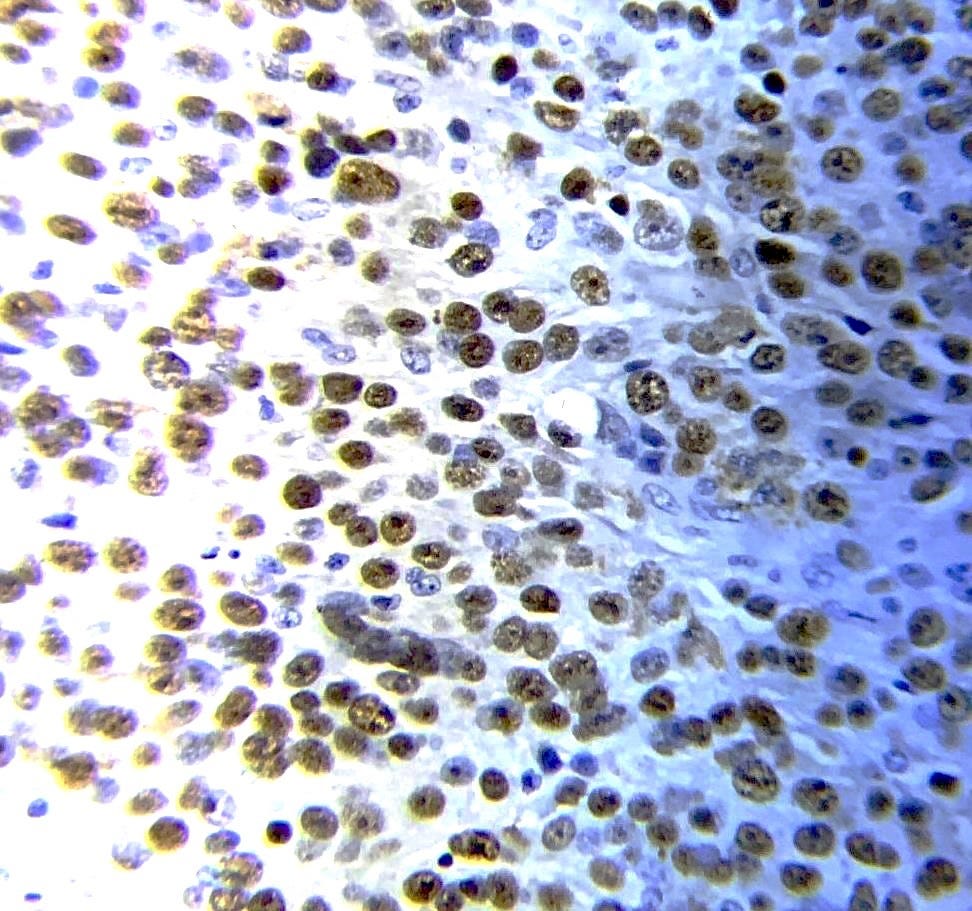
The next essay, part 6f, will discuss other breast cancers without known precursors.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me at https://www.linkedin.com/in/nat-pernick-8967765/ (LinkedIn), npernickmich (Threads and Instagram), natpernick.bsky.social (Bluesky) or @nat385440b (Tribel).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week we post interesting cancer related images of malignancies with diagnoses plus articles of interest. Please also read our CCN essays.
Latest versions of our cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email me at Nat@PathologyOutlines.com - Unfortunately, I cannot provide medical advice.
I also publish Notes at https://substack.com/note. Subscribers will automatically see my Notes.

