Cancer precursor project - breast cancer, part 6f
6 February 2025
Note: I publish 2 blogs - one on politics (daily) and one on cancer and medicine that is scientific and not political (3-4 times per month), which includes this essay - skip if it has no interest to you.
This series in our cancer precursor project discusses how different types of cancer arise - the essays in part 6 discuss breast cancer.
In part 6a, we discussed breast cancer mortality and risk factors and how breast cancer arises. In part 6b, we discussed breast anatomy and histology and started our discussion of the 14 invasive breast cancers with known precursors, which continued in part 6c, part 6d and part 6d1. In part 6e, we began discussing the 31 breast cancers without known premalignant or preinvasive precursors, starting with BRCA associated carcinoma. In part 6f below, we discuss three additional breast cancers without known premalignant or preinvasive precursors, although they may arise from or be associated with benign breast tumors.
How do benign tumors arise in the breast?
The breast is one of the few tissues (also the brain, ovary, testis and uterus) that develops predominantly after birth, during puberty and during pregnancy. Breast tissue contains mammary stem cells which self-renew and differentiate into ductal, myoepithelial and alveolar cells (for milk production) during breast development. Numerous control factors regulate mammary stem cells so they function properly.
Without regulation, stem cells differentiate permanently and do not self-renew. They are regulated by niche signals and growth factors. Niche signals arise from the local tissue microenvironment and include multiple cell types and signaling pathways. They prevent differentiation and allow for stem cell self-renewal. Growth factors promote stem cell growth and proliferation and are inhibited by breast tissue feedback; this prevents unnecessary stem cell proliferation and breast cell growth if an adequate number of breast cells are already present.
Benign breast tumors may arise from genetic mutations in stem cells and progenitor cells that influence these niche signals, growth factors or feedback pathways and cause continued cell growth. Benign tumors typically stop growing because:
Their inability to invade surrounding tissues acts as a growth constraint.
They may be surrounded by a fibrous capsule, which also acts as a barrier to further growth.
They typically have normal cell division mechanisms, which causes them to stop dividing when they reach a certain size.
Niche signaling may only operate within a limited physical area - beyond that, the cells may differentiate and stop dividing.
Why do these benign tumors occasionally transform into malignant tumors?
Stem or progenitor cells may become neoplastic (i.e. grow excessively) due to cancer risk factors, random errors and other factors causing disturbances in the control systems that keep networks in check. There are two possible pathways for benign tumors to become malignant:
The control systems, although disturbed, are initially capable of keeping benign tumors from acquiring invasive properties. Later, the stem or progenitor cells acquire additional mutations that disrupt cell cycle control, causing cells to divide excessively and gain invasive properties. In this pathway, some tumors are initially benign based on either a biopsy or a subsequent excision that shows a benign component.
The changes above occur simultaneously with malignancy associated changes such that the malignancy resembles a benign tumor but the benign tumor never actually existed.
Benign tumors may have other factors associated with subsequent malignancy:
Benign tumors may have areas of atypia or rapid growth that make them more likely to transform (but they may also transform with bland histologic features).
Benign tumors may have intra-tumor genetic heterogeneity, causing clonal selection in the progression to malignancy.
Benign tumors may have a different immune profile from malignant tumors which may cause malignant transformation when disrupted. For example, pleomorphic adenoma of the salivary gland transforms to carcinoma ex pleomorphic adenoma, possibly due to immunosenescence associated with aging. In the breast, immune system escape may be a factor in breast tumor progression but it has not been associated with progression from benign breast disease.
We now describe three benign breast tumors that occasionally transform into invasive breast cancer.
Adenomyoepithelioma with carcinoma
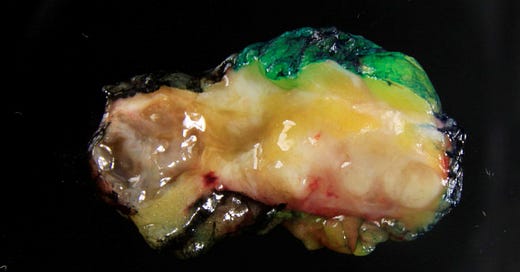
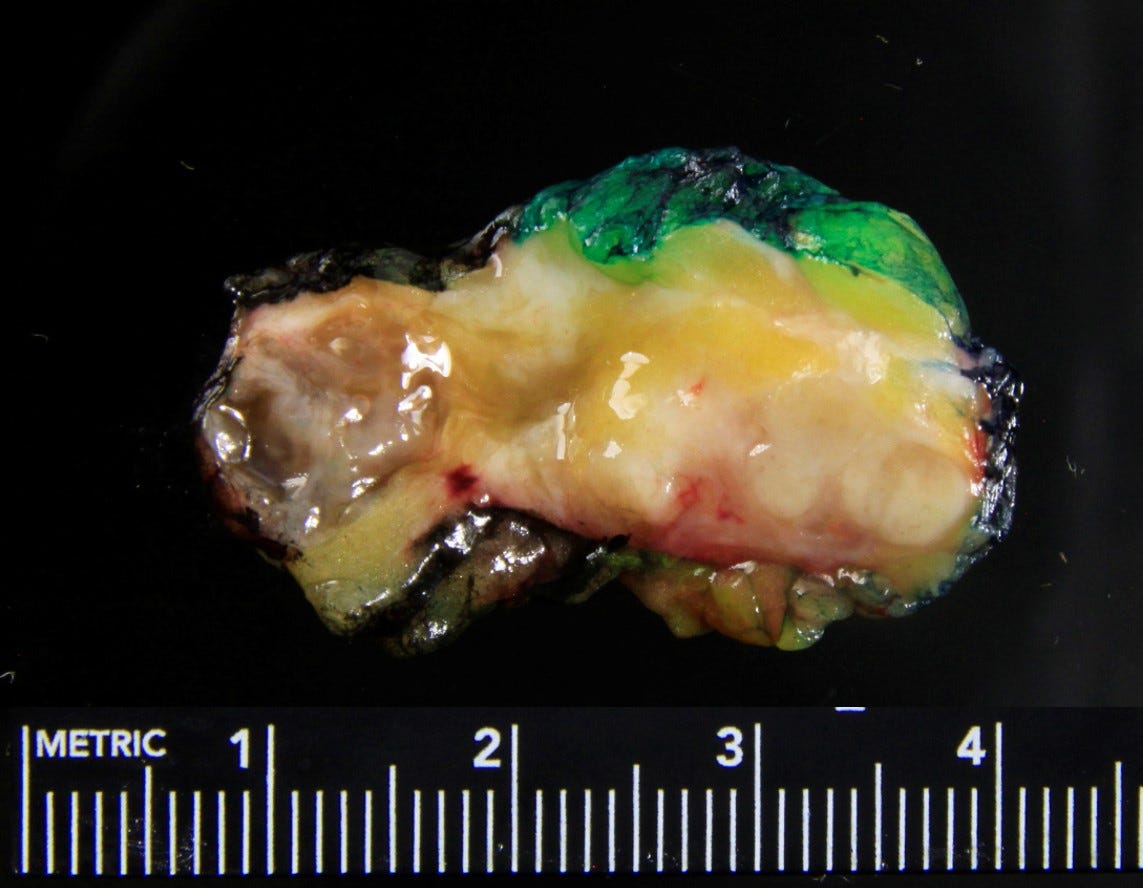
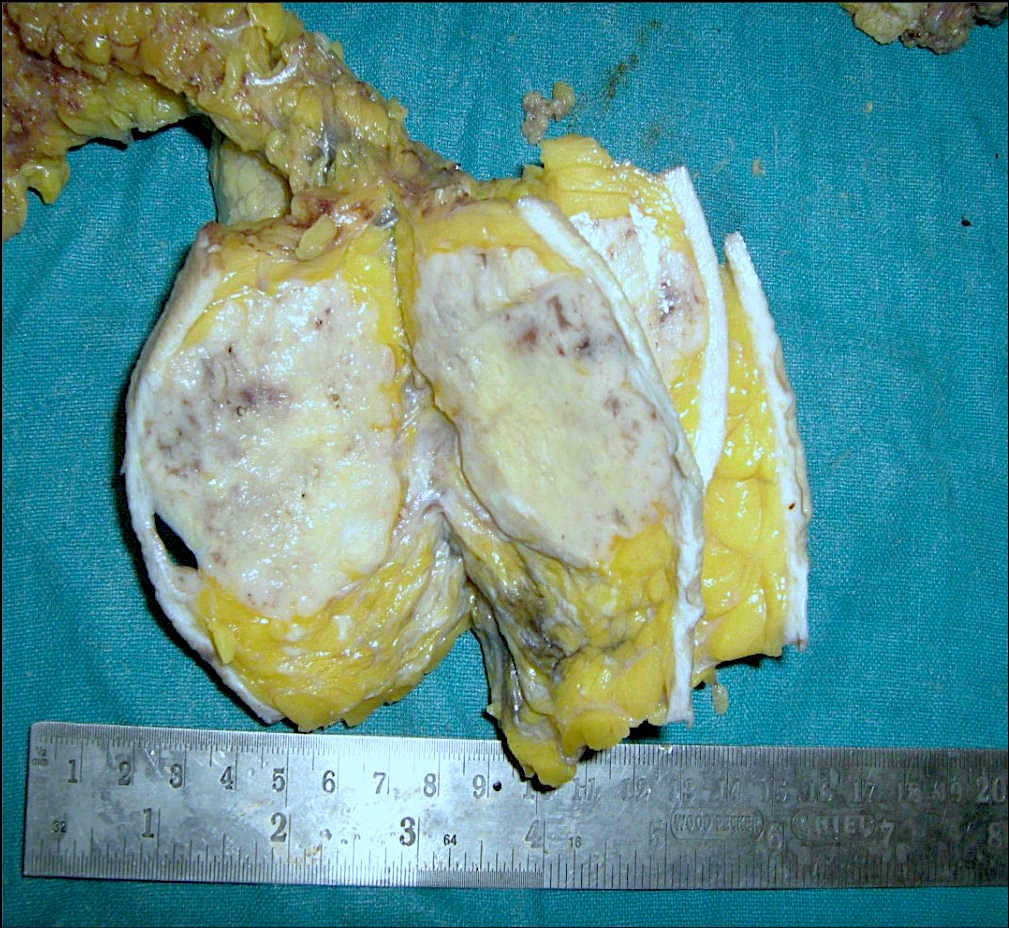
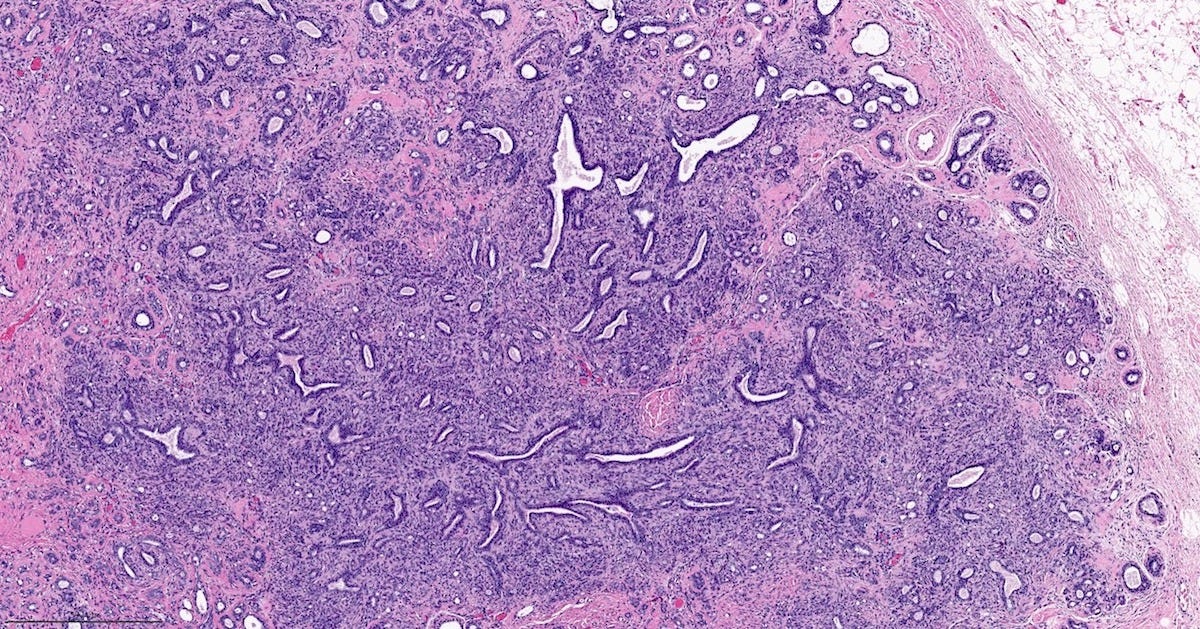
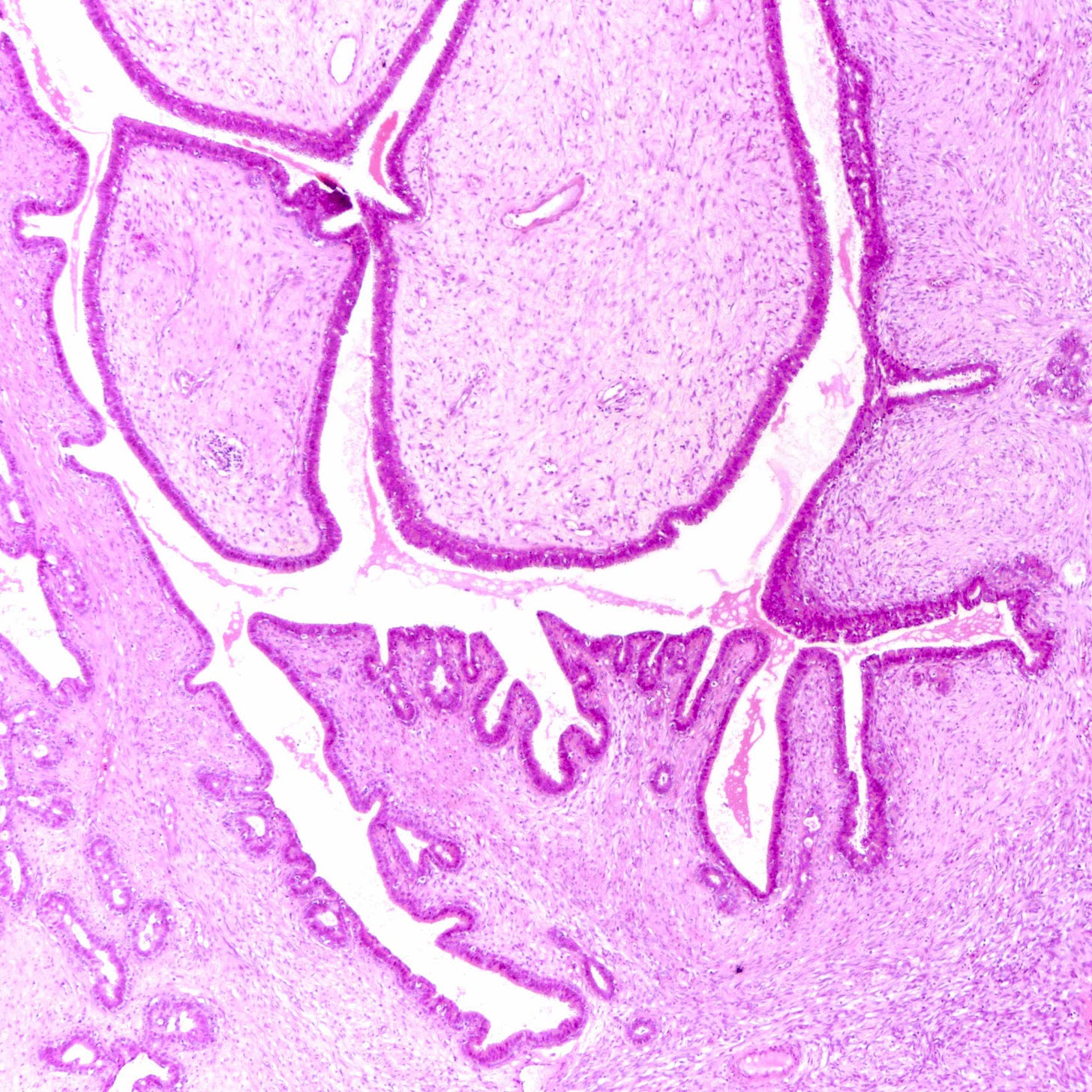
Adenomyoepithelioma with carcinoma is a benign adenomyoepithelioma with malignant transformation. Adenomyoepithelioma is a rare biphasic tumor with epithelial and myoepithelial components. Malignant cases are even rarer. The malignancy can arise in either component and is characterized by an infiltrative growth pattern, nuclear atypia, mitotic activity or necrosis. The benign component is not considered a premalignant precursor because it has no premalignant features and rarely transforms.
Transformation may be caused by various mutations that promote cell proliferation or genomic instability but no cause is identified in many cases.
Adenomyoepithelioma with carcinoma - gross and microscopic images
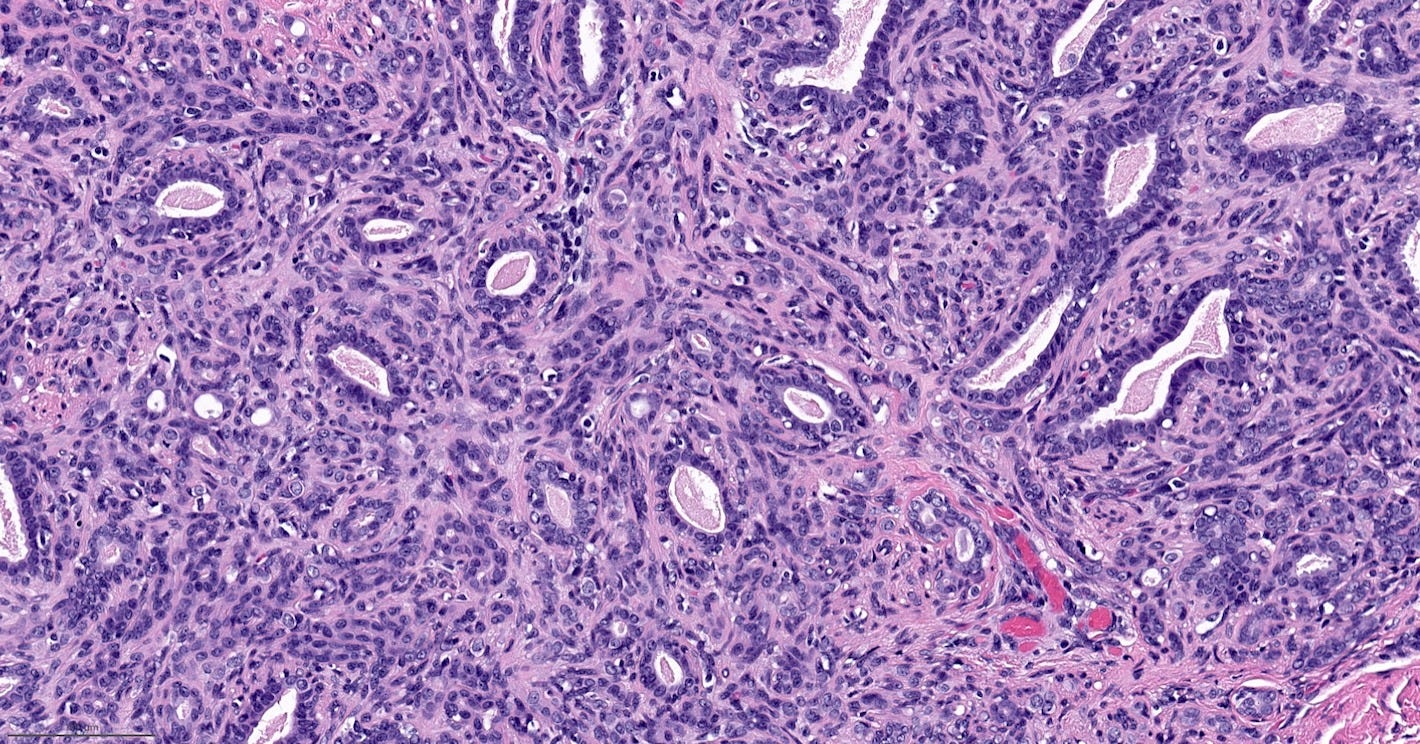
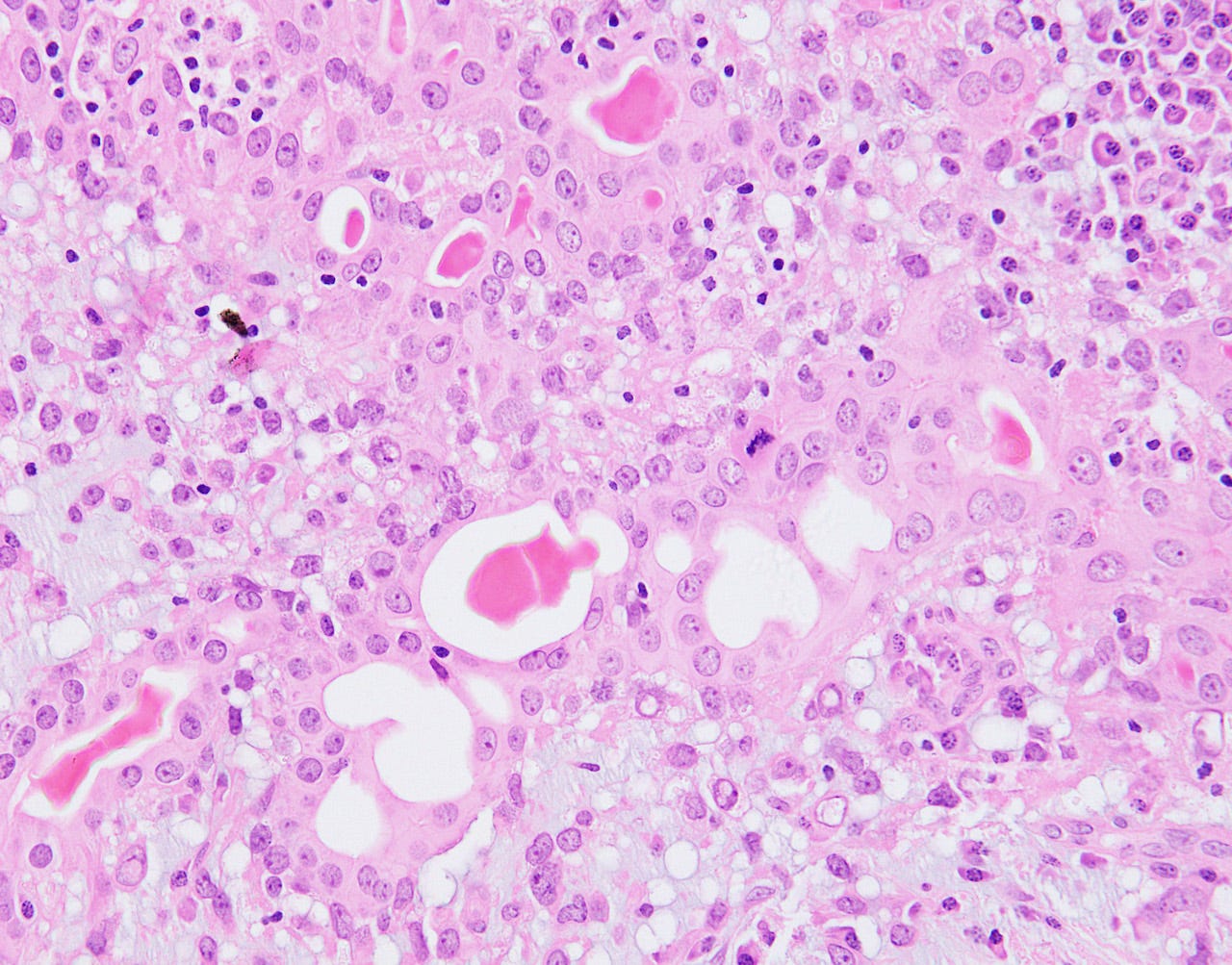
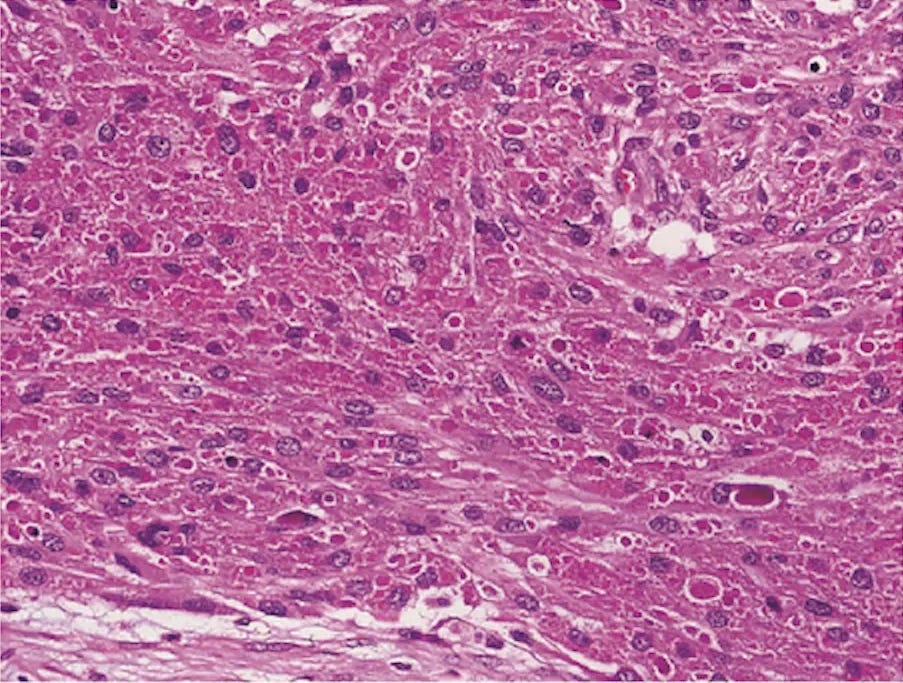
Malignant granular cell tumor
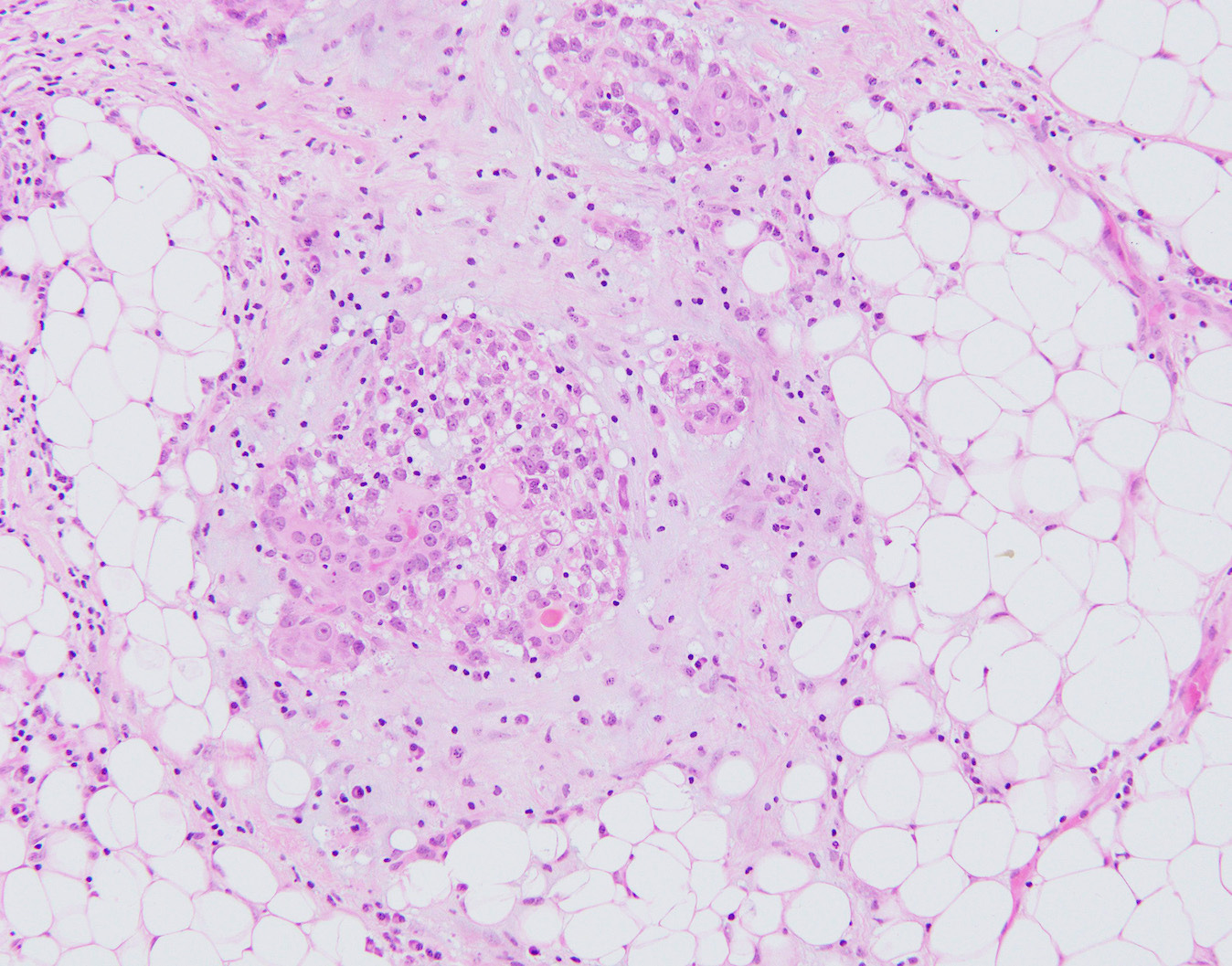
Granular cell tumors are rare, benign, neuroectodermal tumors that arise at many anatomical locations, including the breast. They are derived from Schwann cells in peripheral nerves. Imaging shows an ill defined spiculated lesion mimicking malignancy even in benign cases. Microscopically, granular cell tumors are composed of large epithelioid cells with eosinophilic, granular cytoplasm - the granules are due to abundant lysosomes. Granular cell tumors often show pseudoepitheliomatous hyperplasia, which may be confused with malignancy. By immunohistochemistry, they are positive for S100, SOX10 and CD68.
Most granular cell tumors have mutations in the ATP6AP1 or ATP6AP2 genes located on the X chromosome, which may explain this tumor’s higher prevalence in women. Since these genes encode proteins that regulate the pH of cellular organelles, mutations may cause the accumulation of intracytoplasmic vesicles, including lysosomes.
Only 1-2% of breast granular cell tumors are malignant and fewer than 25 malignant cases in the breast have been reported. Malignant cases may show spindled cells with a high nuclear / cytoplasmic ratio, nuclear pleomorphism, large nucleoli, necrosis and increased mitotic figures, but due to overlap between benign and malignant histology, the presence of metastases remains the only unequivocal sign of malignancy.
Malignant granular cell tumors at all anatomical locations behave similar to malignant peripheral nerve sheath tumors and have a 50% rate of metastasis. Recommended treatment consists of a wider surgical excision. In a 2018 study of 113 malignant granular cell tumors at all sites, overall five and ten year cause specific survival rates were 74.3% and 65.2%. Survival was worse if tumors were 5 cm or larger, if patients had metastases or if the tumor was not completely excised.
Malignant granular cell tumors may arise from:
New mutations in benign tumors, presumably in stem or progenitor cells; one case report documented a benign lesion present for several years which recurred twice after local resection, followed by multiple subcutaneous and lymph node metastases. Alterations in the MAPK, PI3K or TGFβ pathways may be important.
Intra-tumor genetic heterogeneity in benign breast tumors, which may cause clonal selection in the progression to malignancy.
Mutations in stem or progenitor cells that transform directly into a malignant tumor without a preceding benign stage. However, this may be difficult to detect because smaller benign lesions may not be biopsied. In addition, a residual benign granular cell tumor may appear similar enough to the malignant tumor to not be recognized as a distinct entity.
Malignant granular cell tumors of the breast - microscopic images

Due to its rarity, we are using images of malignant granular cell tumor at nonbreast sites from free full text journal articles.


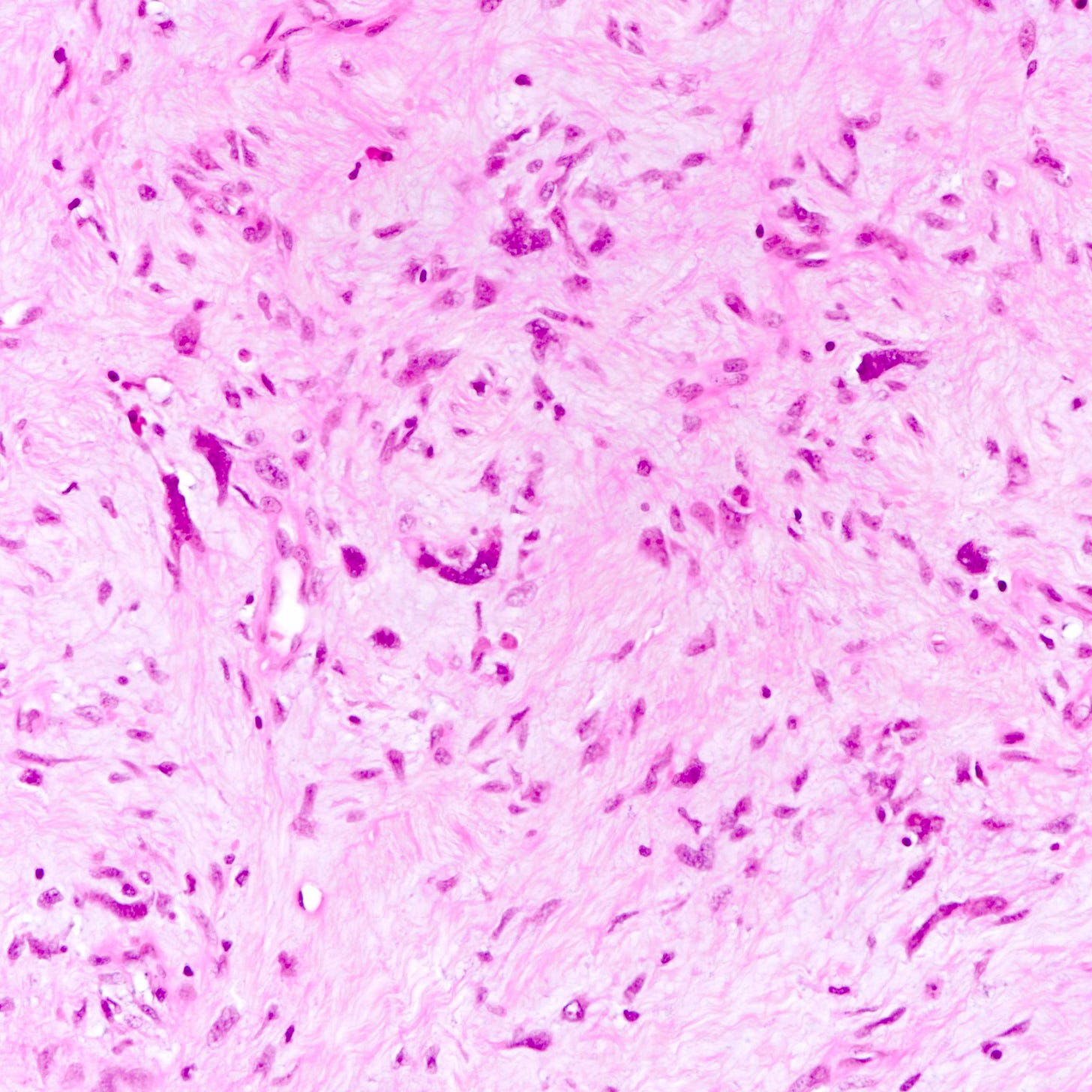
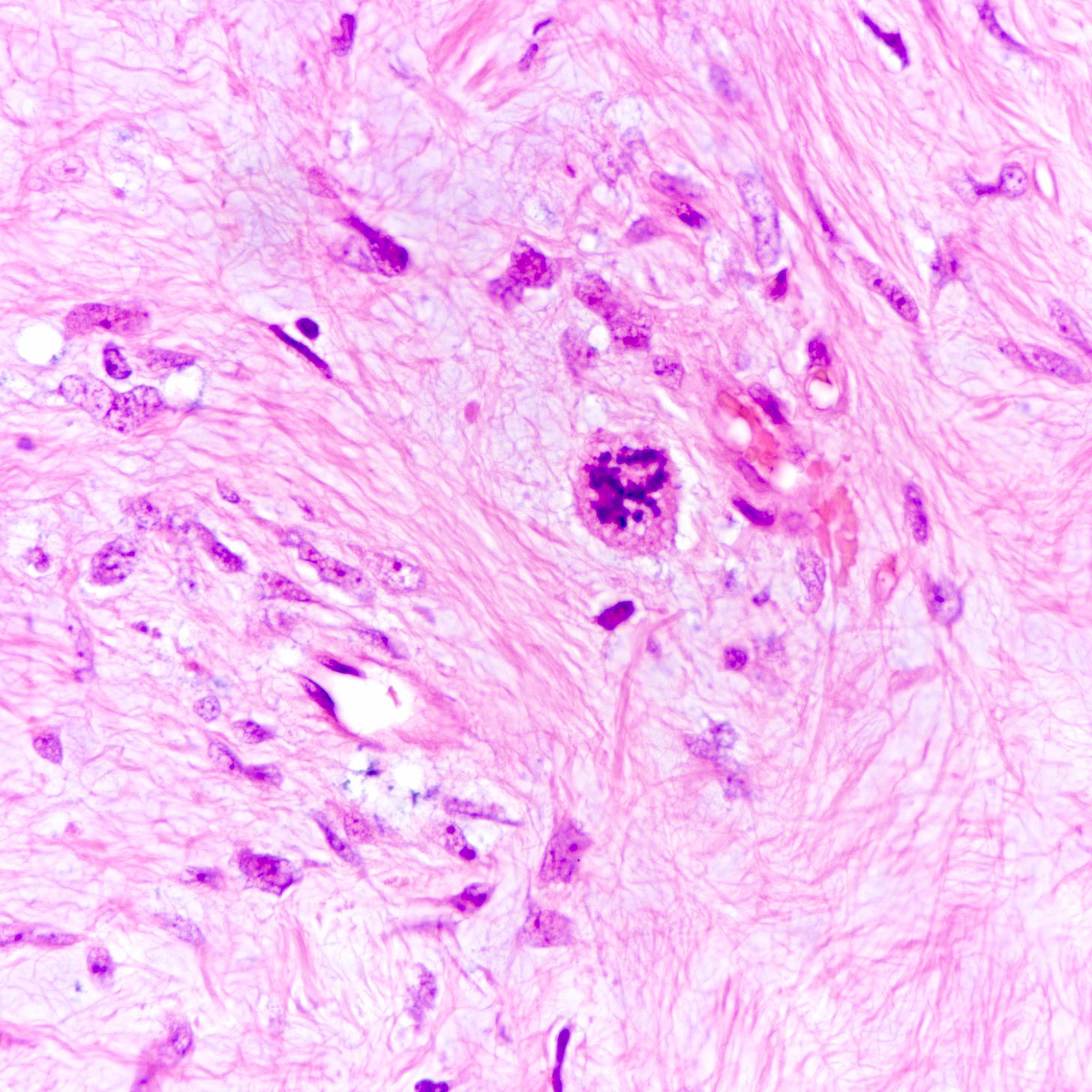
Malignant phyllodes tumor
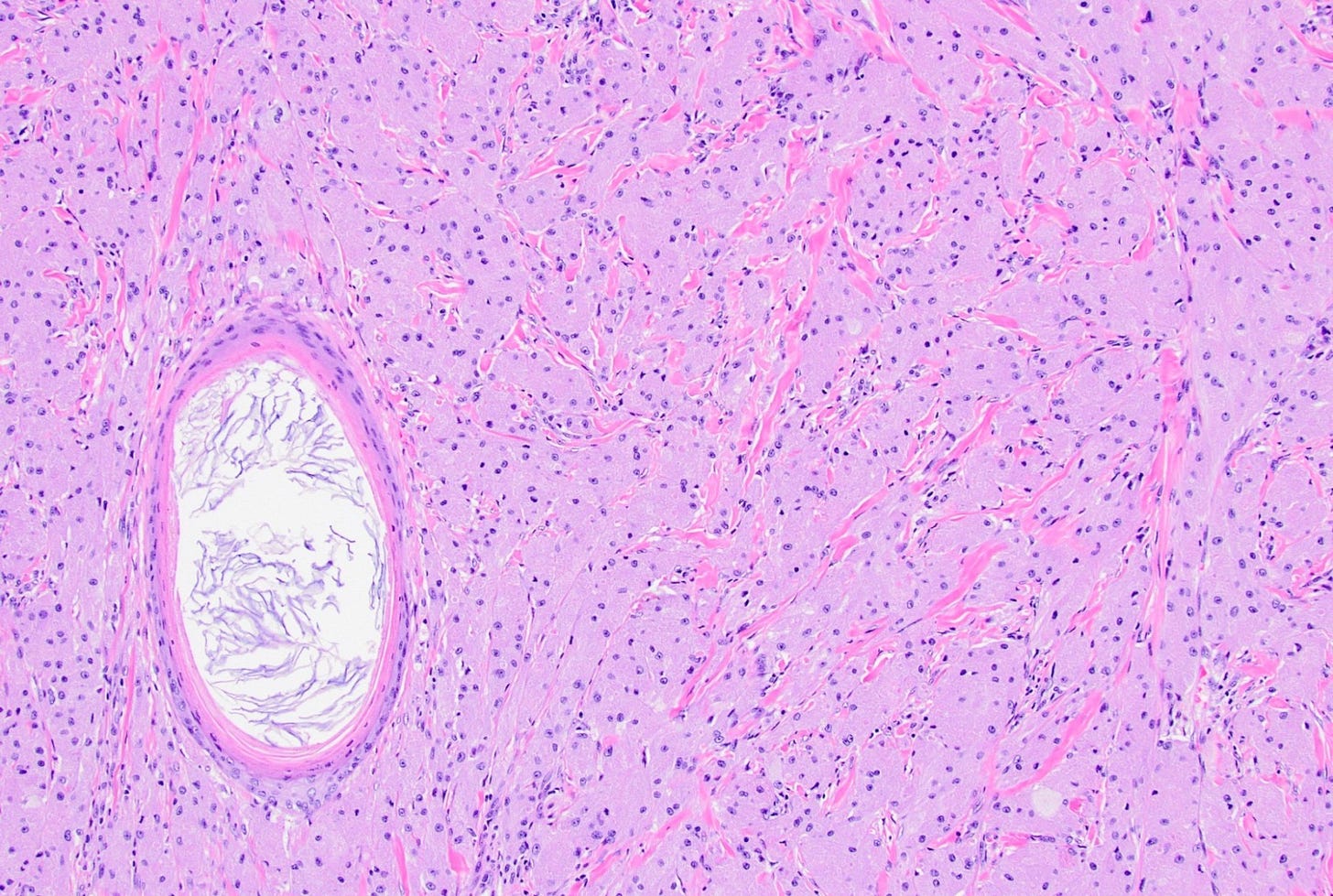
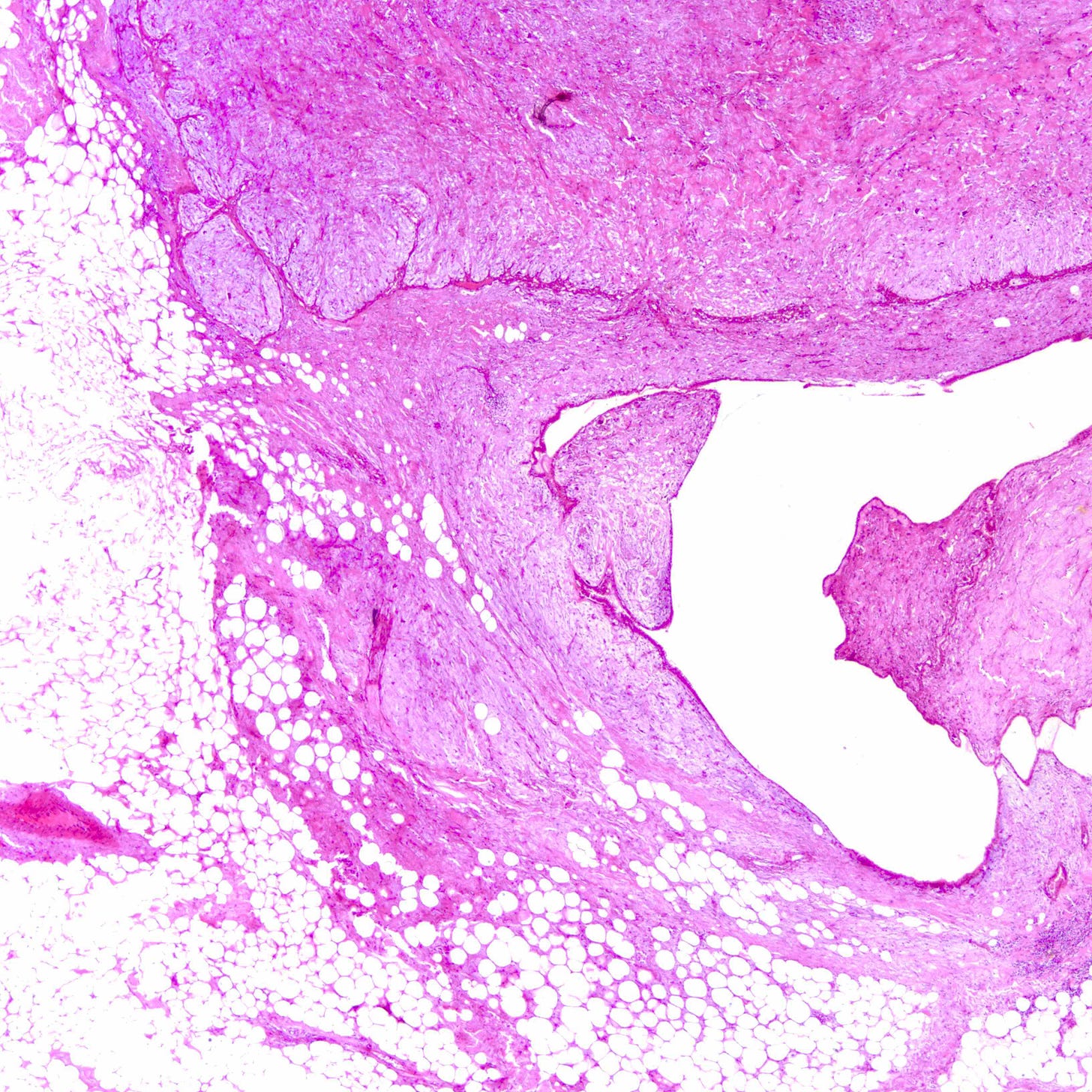
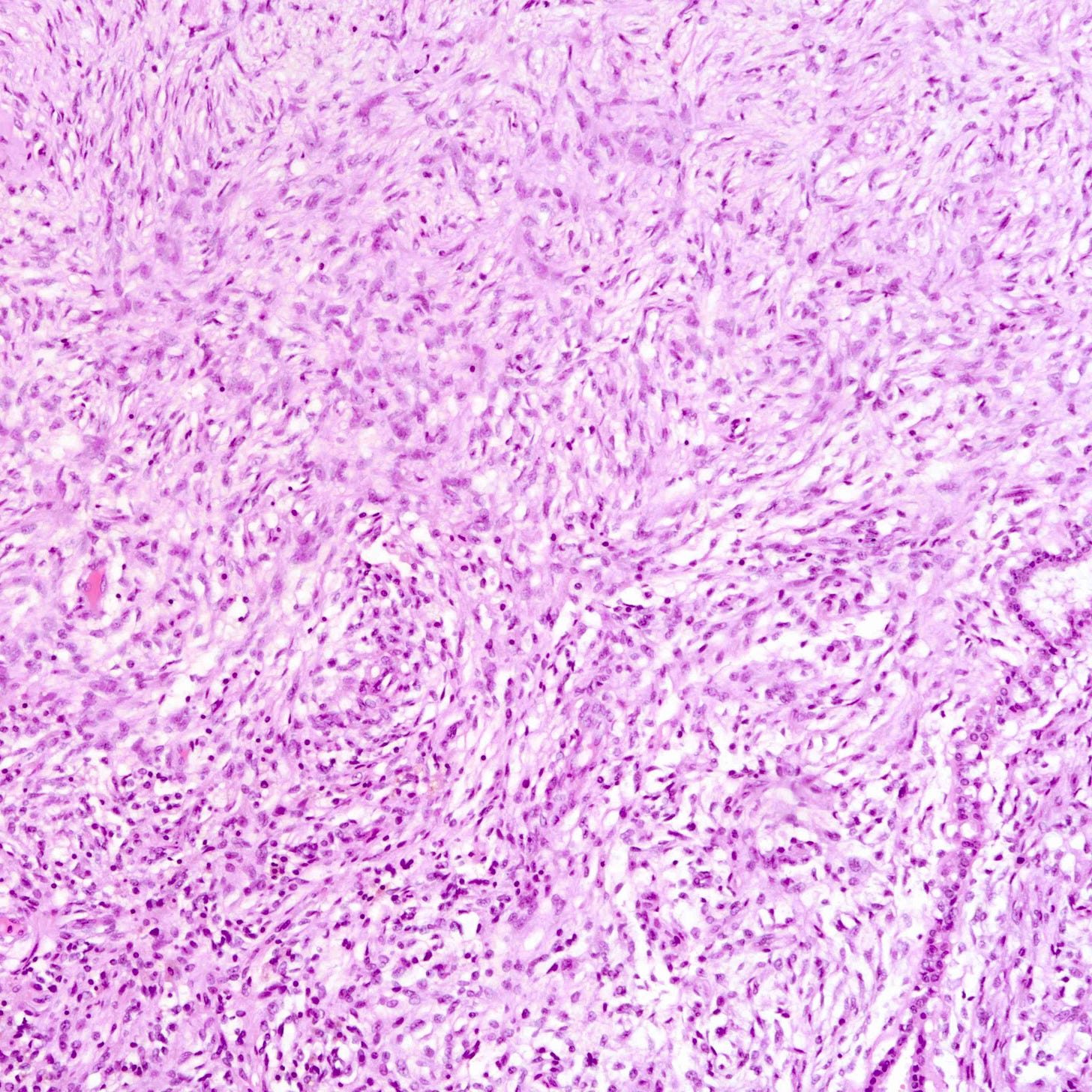
Phyllodes tumor is an uncommon (1% of breast tumors), biphasic fibroepithelial neoplasm with a leaf-like (phylloid) pattern of epithelial and stromal proliferation. It is graded as benign (60-75% of cases), borderline (15-26%) or malignant (8-20%) based on stromal features (atypia, cellularity and overgrowth), the mitotic count and the nature of the tumor border.
All grades of phyllodes tumors may arise due to acquired mutations in the MED12 gene, which are also associated with breast fibroadenoma, pelvic and retroperitoneal leiomyoma and uterine leiomyosarcoma. There may also be an interdependence of growth between the epithelial and stromal components, not yet understood, that explains their complex morphology.
Malignant phyllodes tumors may arise from benign or borderline phyllodes tumors due to cells that undergo malignant transformation or they may arise de novo with no precursor lesion. Of note, malignant transformation is much more common in breast phyllodes tumors than breast fibroadenomas even though both are associated with MED12 mutations.
Malignant phyllodes tumors of the breast - microscopic images
The next essay will discuss other breast cancers without known precursors.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me at https://www.linkedin.com/in/nat-pernick-8967765/ (LinkedIn), npernickmich (Threads and Instagram), natpernick.bsky.social (Bluesky) or @nat385440b (Tribel).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week we post interesting cancer related images of malignancies with diagnoses plus articles of interest. Please also read our CCN essays.
Latest versions of our cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email me at Nat@PathologyOutlines.com - Unfortunately, I cannot provide medical advice.
I also publish Notes at https://substack.com/note. Subscribers will automatically see my Notes.