Cancer precursor project - breast cancer, part 6i
16 March 2025
This weekly medical blog is scientific, not political. I also publish a daily political blog. Skip any essays or parts of essays that you find too technical or are not interested in - I won’t be offended.
This essay is part of my cancer precursor project that discusses how different types of cancer arise. Part 6 discusses breast cancer and its 45 separate types:
Part 6a: breast cancer mortality and risk factors and how breast cancer arises.
Invasive breast cancers with premalignant precursors:
Part 6b: breast anatomy and histology, infiltrating ductal carcinoma of no special type, lobular carcinoma and pleomorphic lobular carcinoma.
Part 6c: cribriform carcinoma, secretory carcinoma, neuroendocrine-small cell carcinoma and male invasive breast carcinoma.
Part 6d: tubular carcinoma, triple negative breast cancer, acinic cell carcinoma and metaplastic carcinoma: squamous cell carcinoma.
Part 6d1: encapsulated papillary carcinoma with invasion, solid papillary carcinoma with invasion and intraductal papillary carcinoma with invasion.
Invasive breast cancers without premalignant or preinvasive precursors:
Part 6e: BRCA associated carcinoma.
Part 6f: adenomyoepithelioma with carcinoma, malignant granular cell tumor and malignant phyllodes tumor.
Part 6g: glycogen rich carcinoma, lipid rich carcinoma, oncocytic carcinoma and sebaceous carcinoma.
Part 6h: apocrine carcinoma and invasive micropapillary carcinoma. It also summarizes all major DCIS subtypes and their relationship to invasive breast carcinoma.
Part 6i discusses mucin containing carcinomas of the breast without premalignant or preinvasive precursors and how they arise:
We also briefly discuss other mucinous lesions, either benign (mucocele-like lesion), preinvasive (mucinous ductal carcinoma in situ), variants of invasive carcinoma discussed previously (cribriform carcinoma, infiltrating ductal carcinoma of no special type, invasive lobular carcinoma with extracellular mucin, invasive micropapillary carcinoma and solid papillary carcinoma) and metastases to the breast.
Mucinous carcinoma
Mucinous carcinoma is an invasive breast carcinoma with a mucinous component that comprises > 90% of the tumor and has a favorable prognosis. Only 2% of breast carcinomas are pure mucinous carcinoma, although 3 - 4% have some mucinous component (the rest are < 90% of the tumor). Patients with pure mucinous carcinoma are usually older than those with typical breast carcinoma (median 71 years versus 61 years).
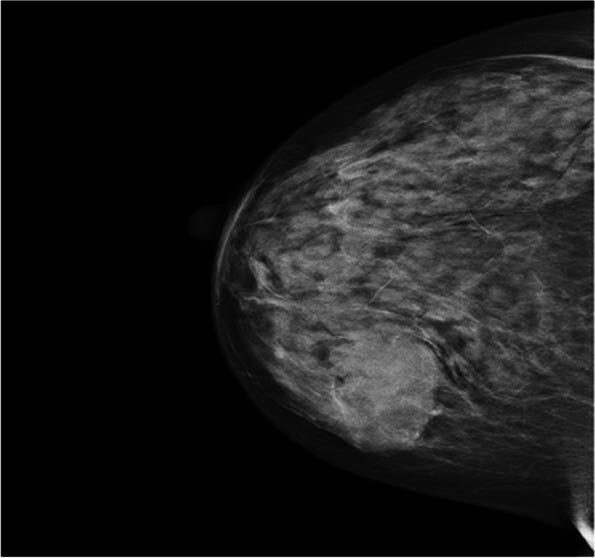
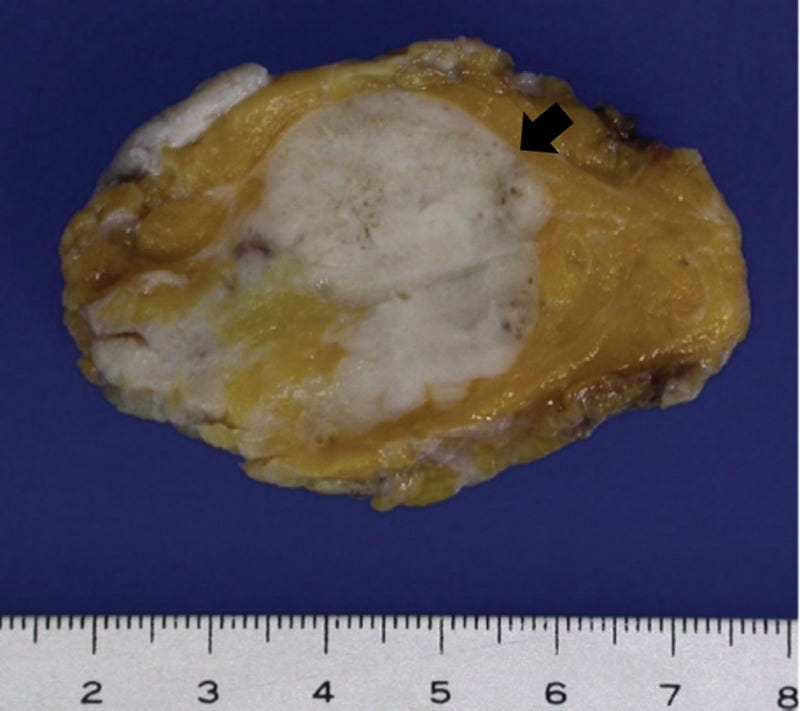
Grossly, these tumors are well circumscribed and variably sized, from < 1 cm to > 20 cm. They have a distinctive, gelatinous cut surface due to the mucin content.
There are two microscopic patterns, but both have a similar prognosis. Type A has small clusters of tumor cells within secreted mucin. Type B has large sheets of tumor cells with neuroendocrine features and mucin within the sheets. Mucinous carcinomas are ER and PR receptor positive and HER2 negative.
Patients with pure mucinous carcinoma have a favorable prognosis with a 10 year survival rate of 90%. Patients tend to have localized disease with only rare involvement of axillary lymph nodes and a low recurrence rate. Treatment is surgical excision and adjuvant hormone therapy. Of note, patients with < 90% mucinous components have a less favorable prognosis.
How mucinous carcinoma arises is unknown. It is associated with prolonged estrogen exposure (early menarche, nulliparity or exogenous hormone use), genetics and diet. These tumors lack the genetic changes (gains of 1q, loss of 16q and PIK3CA mutations) typical of other ER+, PR+, low grade neoplasms.
There are no known precursors of mucinous carcinoma. Due to its distinction from other low grade neoplasms, it likely has a different transformation pathway. We speculate that mucinous carcinoma may arise due to the hijacking of mucin production as a vehicle for malignant transformation, analogous to how glycogen rich carcinoma arises, as discussed in part 6g.
Mucinous carcinoma - radiologic, gross, microscopic and electron microscopy images
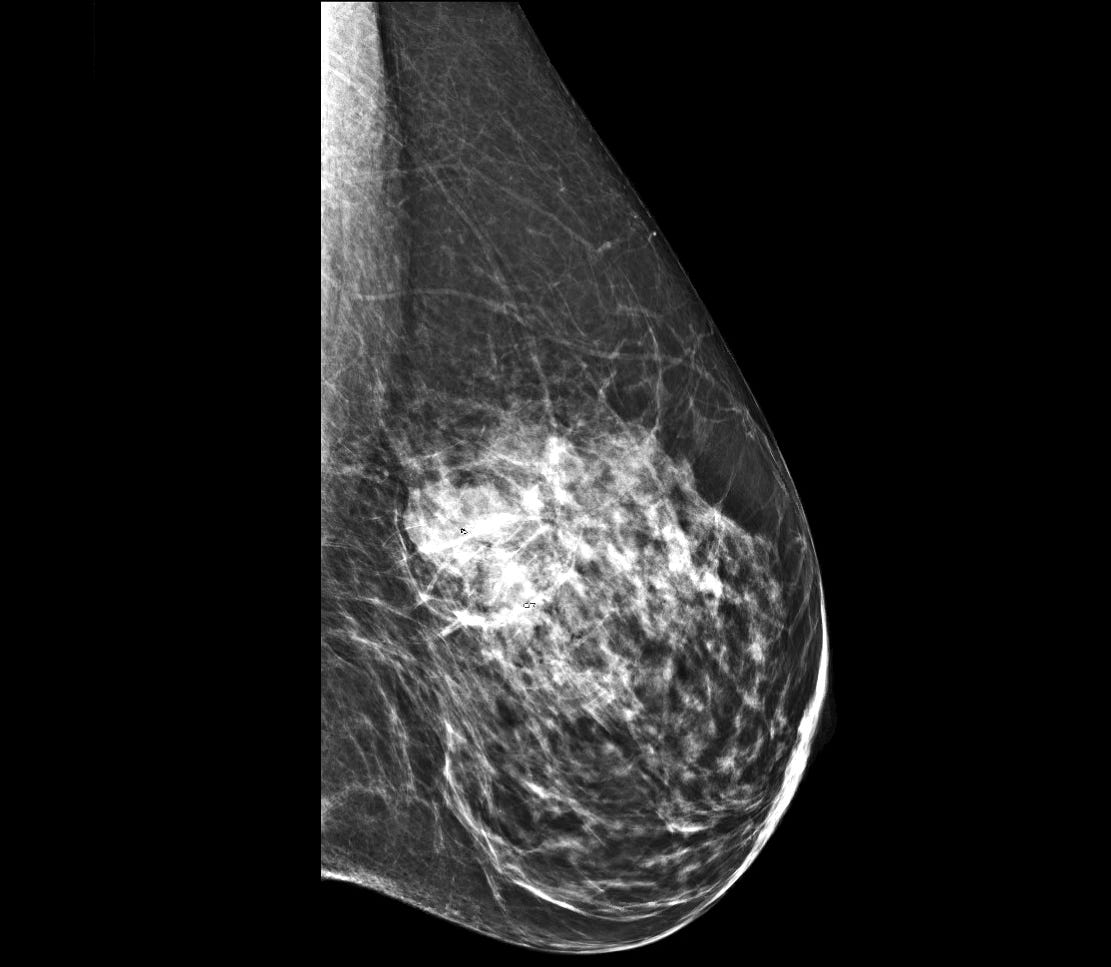
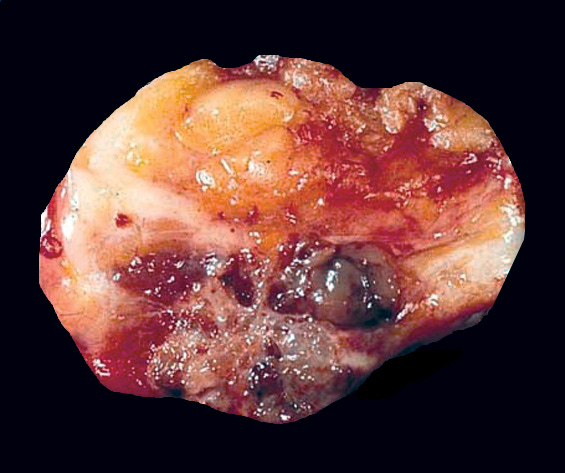
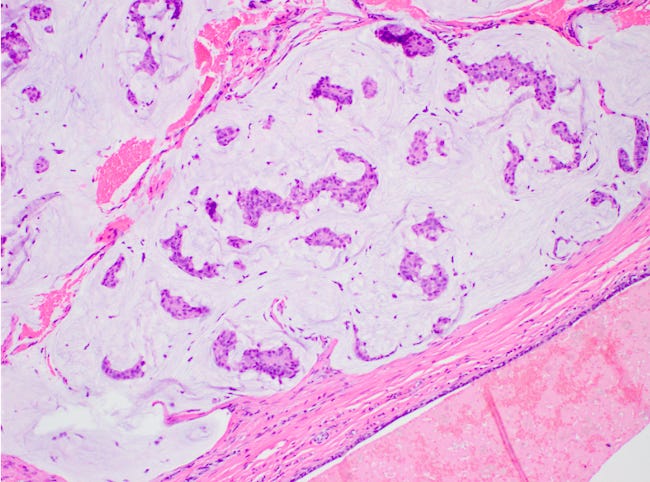
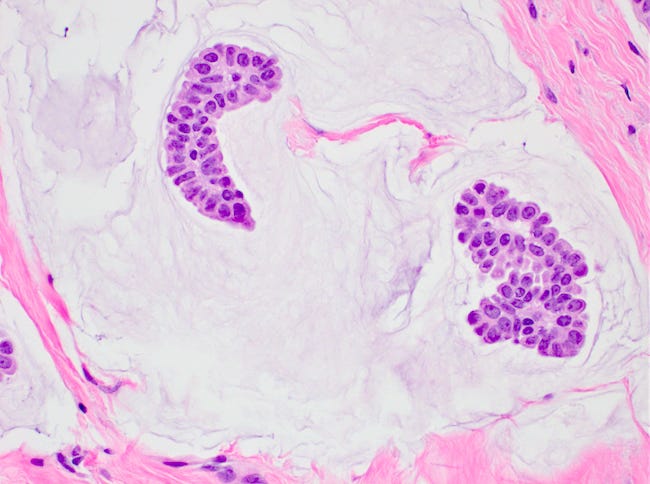
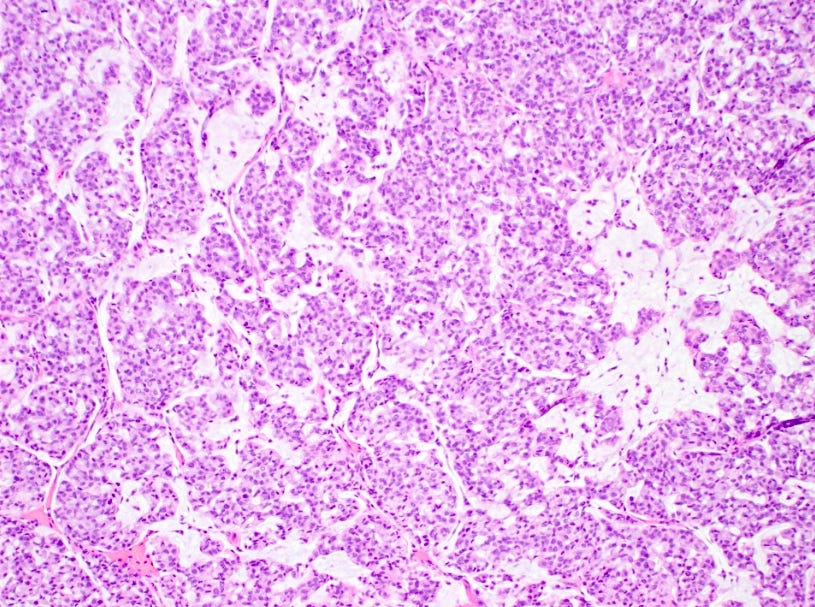
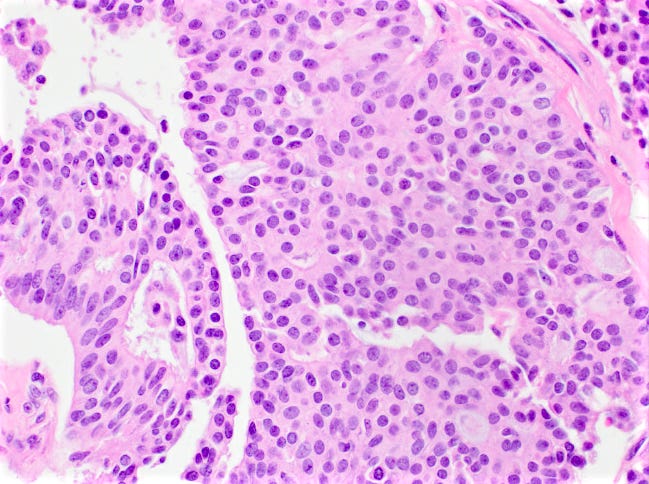
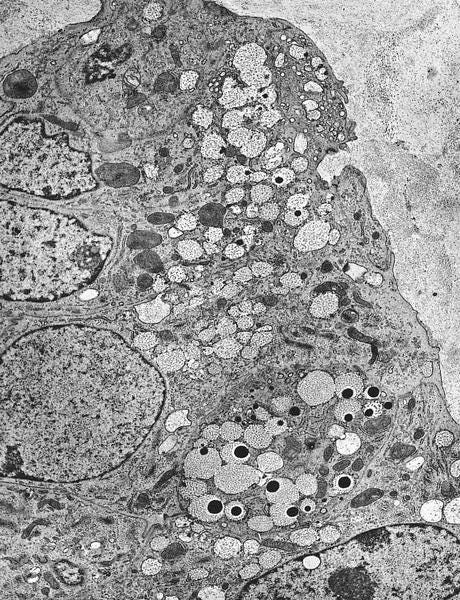
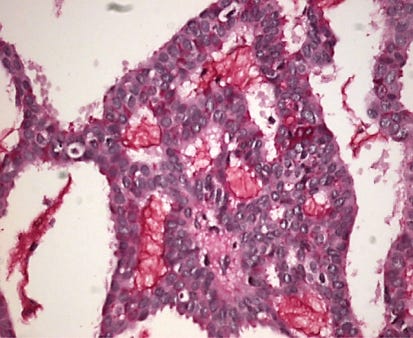
Mucinous cystadenocarcinoma
Mucinous cystadenocarcinoma, first described in 1998 is a very rare primary breast tumor with fewer than 50 cases reported. It is part of the 2019 WHO Breast Classification of Tumours,
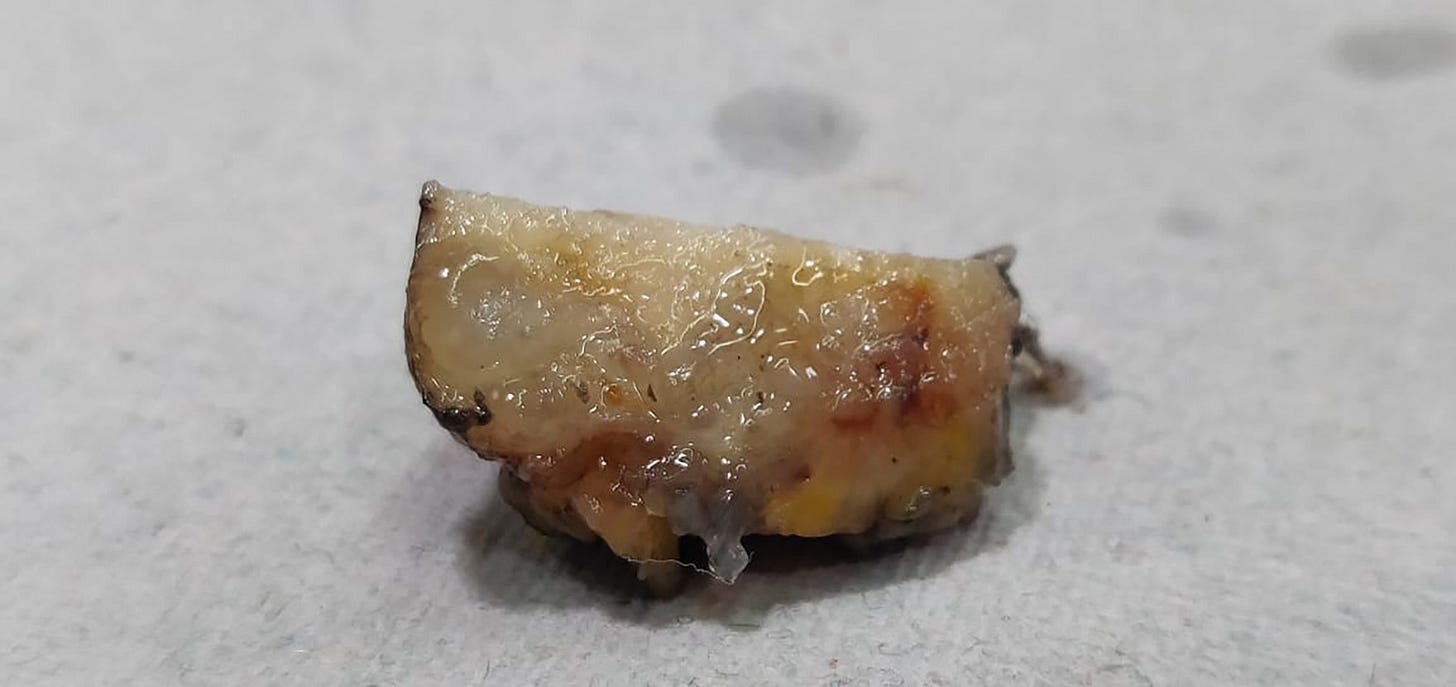
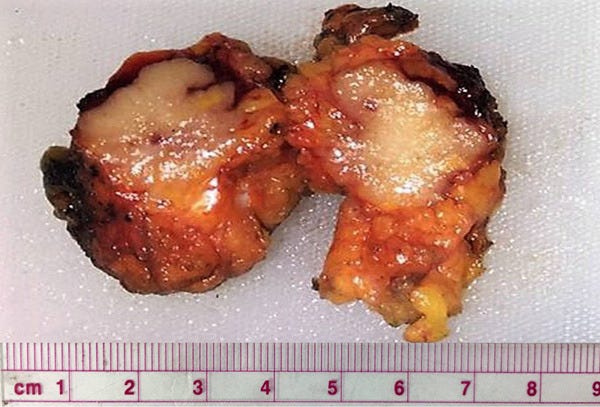
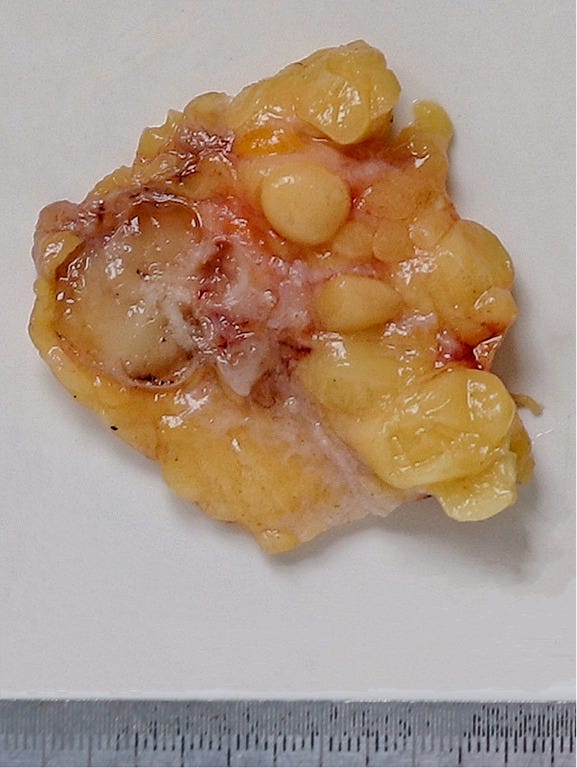
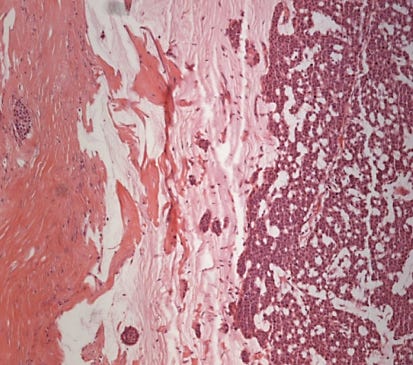
It occurs in women ages 41 to 96 years. It ranges from 1 - 19 cm and is typically grossly multicystic but may appear solid. Microscopically, it has cystic spaces lined by tall stratified columnar cells with papillary structures and tufting. The nuclei are basal with variable atypia. There is abundant intracytoplasmic and extracytoplasmic mucin. In contrast, mucinous carcinoma has only extracellular mucin and signet ring cell carcinoma has only intracellular mucin.
Mucinous cystadenocarcinoma is usually triple negative (i.e., estrogen receptor (ER) negative, progesterone receptor (PR) negative and HER2 negative). It appears to have a better prognosis than other triple negative breast carcinomas but reported followup has been limited. It is usually negative for nodal metastases at presentation. It must be differentiated from metastases of similar appearing tumors in the ovary, pancreas and appendix - clinical correlation and TRPS1 immunohistochemistry may be useful. It must also be differentiated from the other mucinous lesions discussed below.
Mucinous cystadenocarcinoma does not appear to have a premalignant precursor. How it arises is unknown but suggested theories are that: (a) it arises from mucinous metaplasia and macrocystic transformation of ordinary breast carcinoma; (b) it arises from intraductal papillary carcinoma with mucinous metaplasia and extracellular mucin production, leading to cystic dilation of the lumen with loss of myoepithelial cells and invasion of the adjacent stroma; and (c) cases accompanied by DCIS arise from mucinous metaplasia of DCIS epithelial cells accompanied by loss of ER and PR expression.
None of these theories explain how mucinous cystadenocarcinoma became triple negative yet has a good prognosis or how it contains both intra- and extracytoplasmic mucin. Perhaps, as with mucinous carcinoma of the breast, mucinous cystadenocarcinoma arises due to the hijacking of mucin production as a vehicle for malignant transformation.
Of note, mucin production by itself has a variable association with prognosis. As noted above, pure mucinous carcinoma of the breast has a favorable prognosis. Pancreatic colloid (mucinous noncystic) carcinoma also has a favorable prognosis, attributed to extracellular mucin limiting tumor spread and having tumor suppressor activity. However, mucinous (colloid) carcinomas of the ovary and appendix have a worse prognosis than nonmucinous tumors at these sites.
Mucinous cystadenocarcinoma - radiologic, gross, microscopic and cytology images
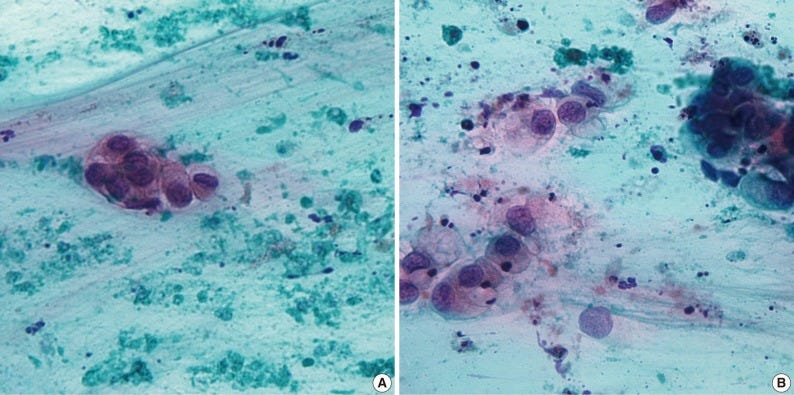
Mucoepidermoid carcinoma
Mucoepidermoid carcinoma of the breast is very rare (0.2 - 0.3 % of all breast malignancies, fewer than 100 cases reported), although it is the most common malignancy of the salivary gland. The first cases in the breast were reported in 1979. It is predominantly triple negative with a relatively favorable prognosis, in contrast to typical triple negative cases.
Regardless of location, mucoepidermoid carcinoma is characterized by a mixture of basaloid, mucinous, squamous and intermediate neoplastic cells arranged in solid and cystic patterns.
Tumor grade is the most important predictor of prognosis. Low grade tumors, which are most common, tend to be more cystic and have bland nuclear features. They have a favorable clinical outcome with no deaths reported. High grade tumors, which are uncommon, are more solid. They have marked nuclear atypia, necrosis and brisk mitotic figures but may have less visible mucin. They are associated with axillary nodal and distant metastases.
Immunohistochemistry for GATA3 and mammaglobin distinguish these breast malignancies from metastatic mucoepidermoid carcinoma of the salivary glands (GATA3 and mammaglobin are positive in the breast and negative in salivary glands). Although mucoepidermoid carcinoma of the breast is typically negative for ER, PR and HER2, some recent cases were ER positive.
Low grade tumors may be treated with limited surgery (breast conservation) and possibly sentinel node biopsy. High grade tumors are typically managed through mastectomy and axillary lymph node dissection.
Breast tissue and major salivary glands have structural similarities that generate comparable neoplastic lesions. Both are exocrine glands composed of tubules and acini derived from the embryonic ectoderm. Both have luminal epithelial cells surrounded by myoepithelial cells.
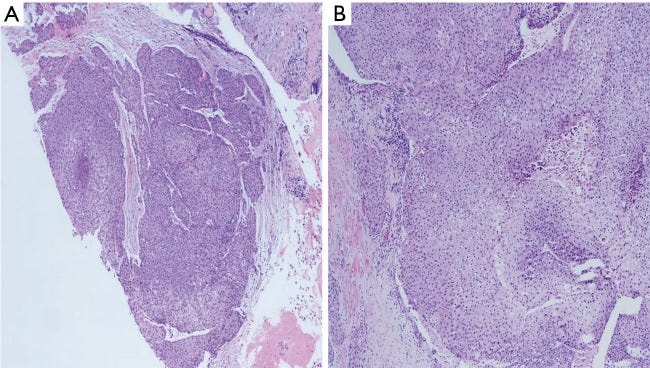
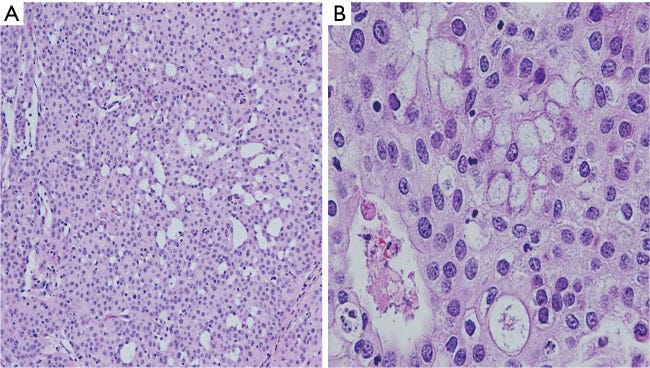
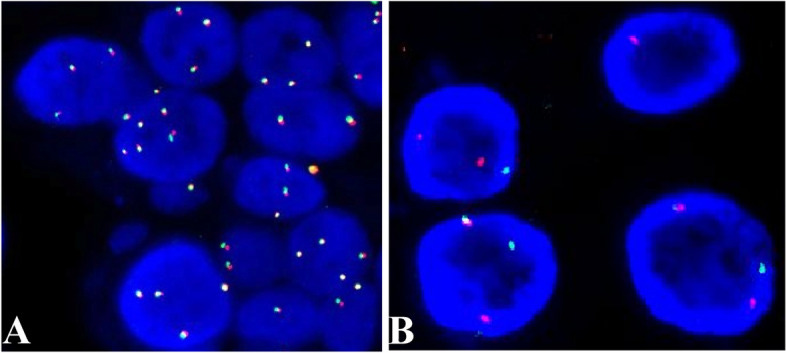
In the salivary glands, mucoepidermoid carcinoma may arise from pluripotent reserve cells of the excretory ducts, which can differentiate into squamous, columnar and mucous cells. The most frequent molecular alteration involves rearrangements of the MAML2 gene, which causes the overproduction of the AREG and EGFR proteins and is a key driver for developing and maintaining mucoepidermoid carcinoma. In the breast, it is unknown how mucoepidermoid carcinoma arises. There is no known precursor lesion. Breast mucoepidermoid carcinoma may or may not have MAML2 rearrangements, but it does overproduce the AREG and EGFR proteins through a different pathway. At the genetic level, breast mucoepidermoid carcinoma resembles other invasive breast carcinomas more than salivary gland tumors.
Mucoepidermoid carcinoma - radiologic, gross, microscopic and molecular images
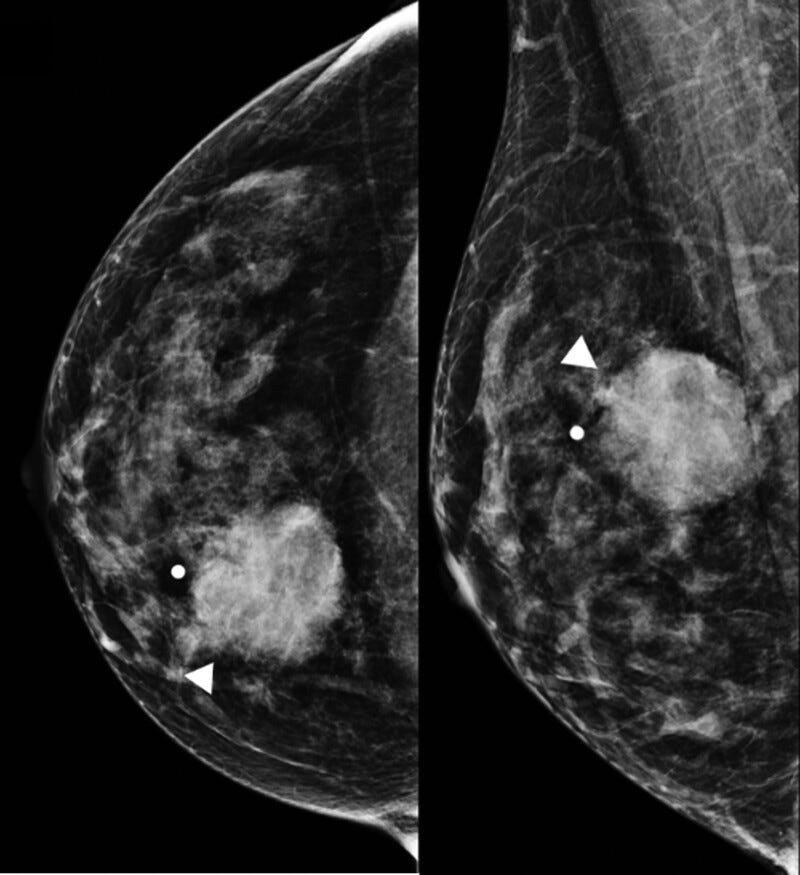
We now describe other mucinous lesions of the breast, for completeness:
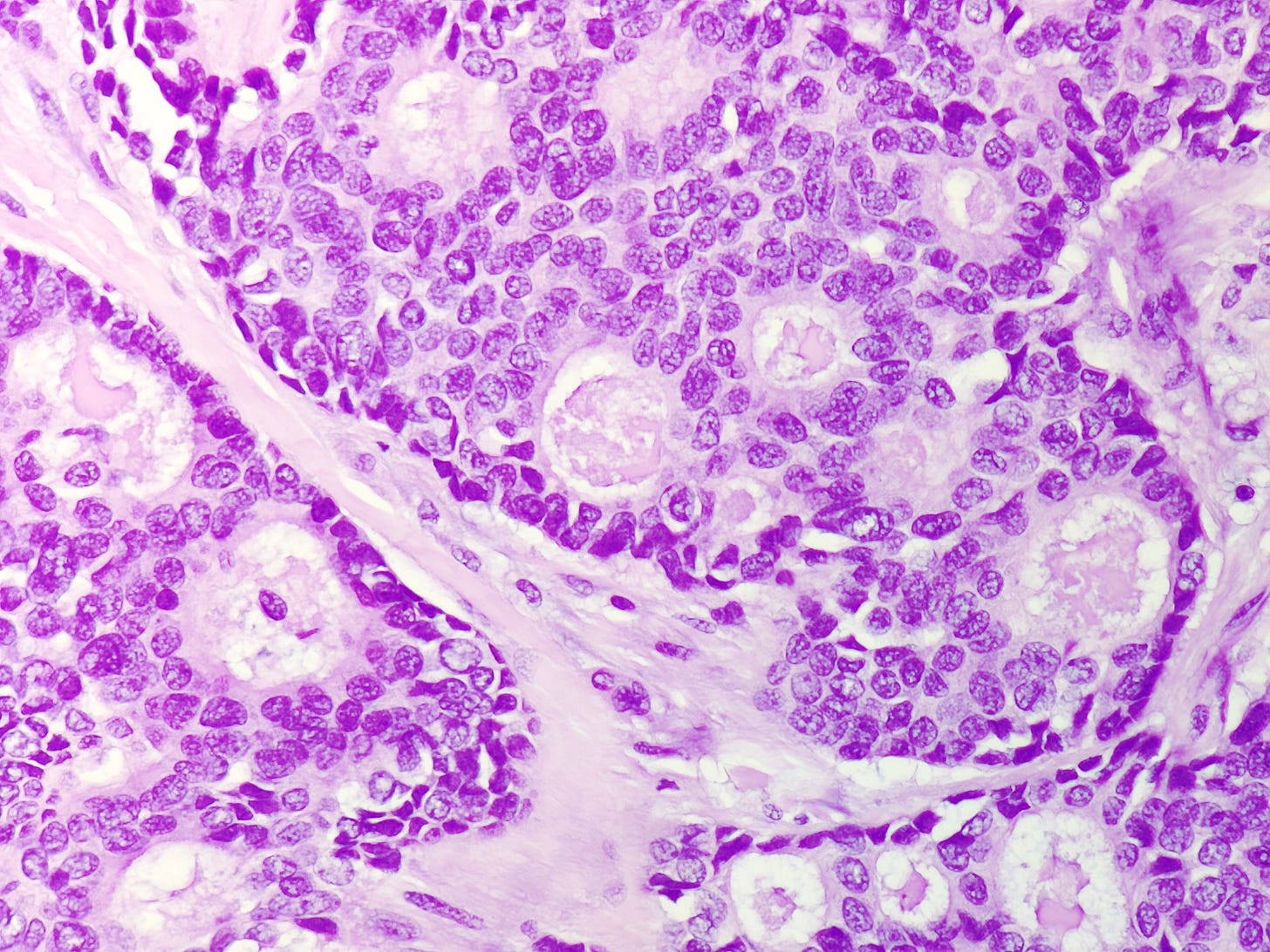
Cribriform carcinoma, as previously discussed, may contain intraluminal mucin but has a distinct cribriform pattern:
Invasive ductal carcinoma, no special type, as previously discussed, may have features of pure mucinous carcinoma but with less than the required 90% for this diagnosis.
Invasive lobular carcinoma, as previously discussed, may have signet ring cells in which the intracytoplasmic mucin displaces the nuclei to give a distinct appearance:
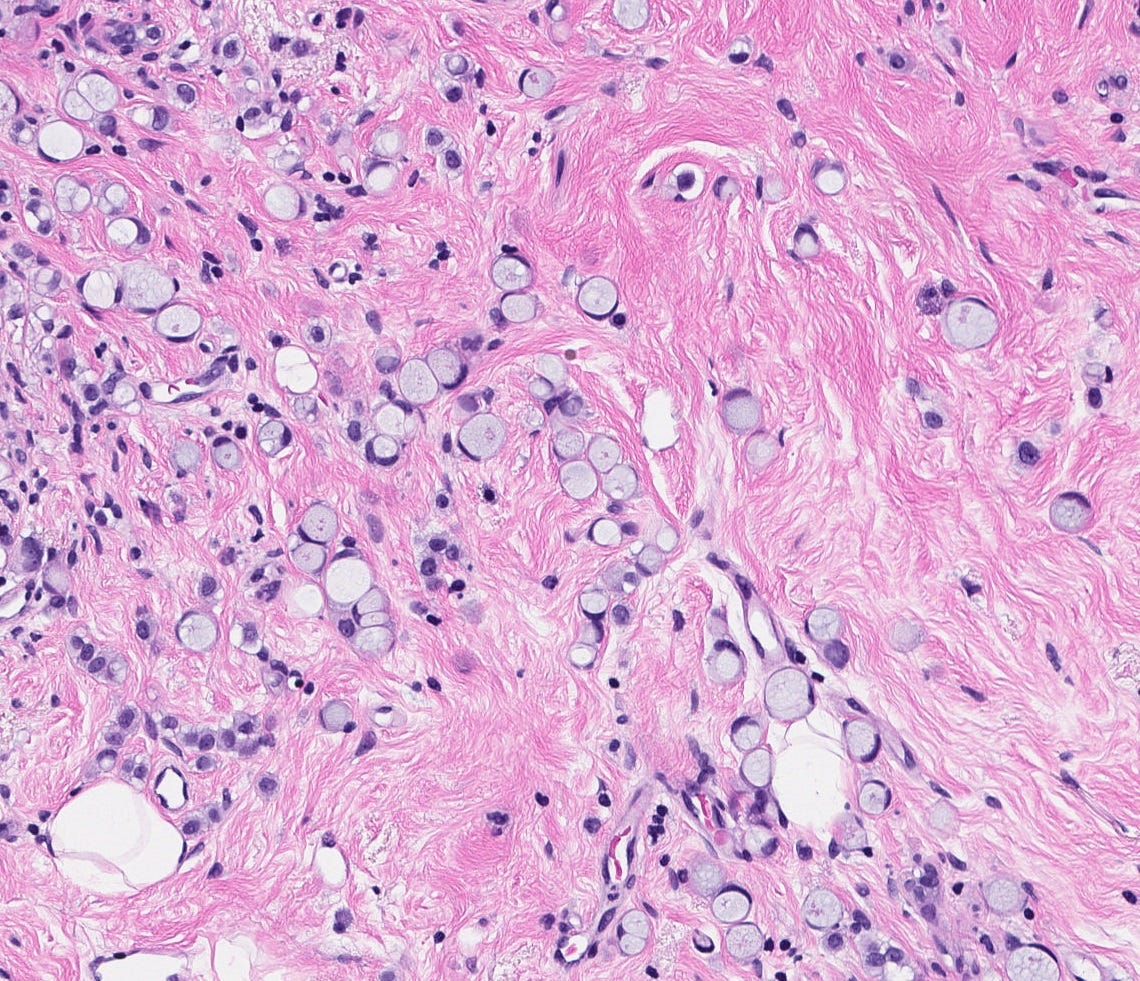
Invasive micropapillary carcinoma, as previously discussed, may have nests of tumor cells floating in mucin (this may also be considered a variant of mucinous carcinoma):
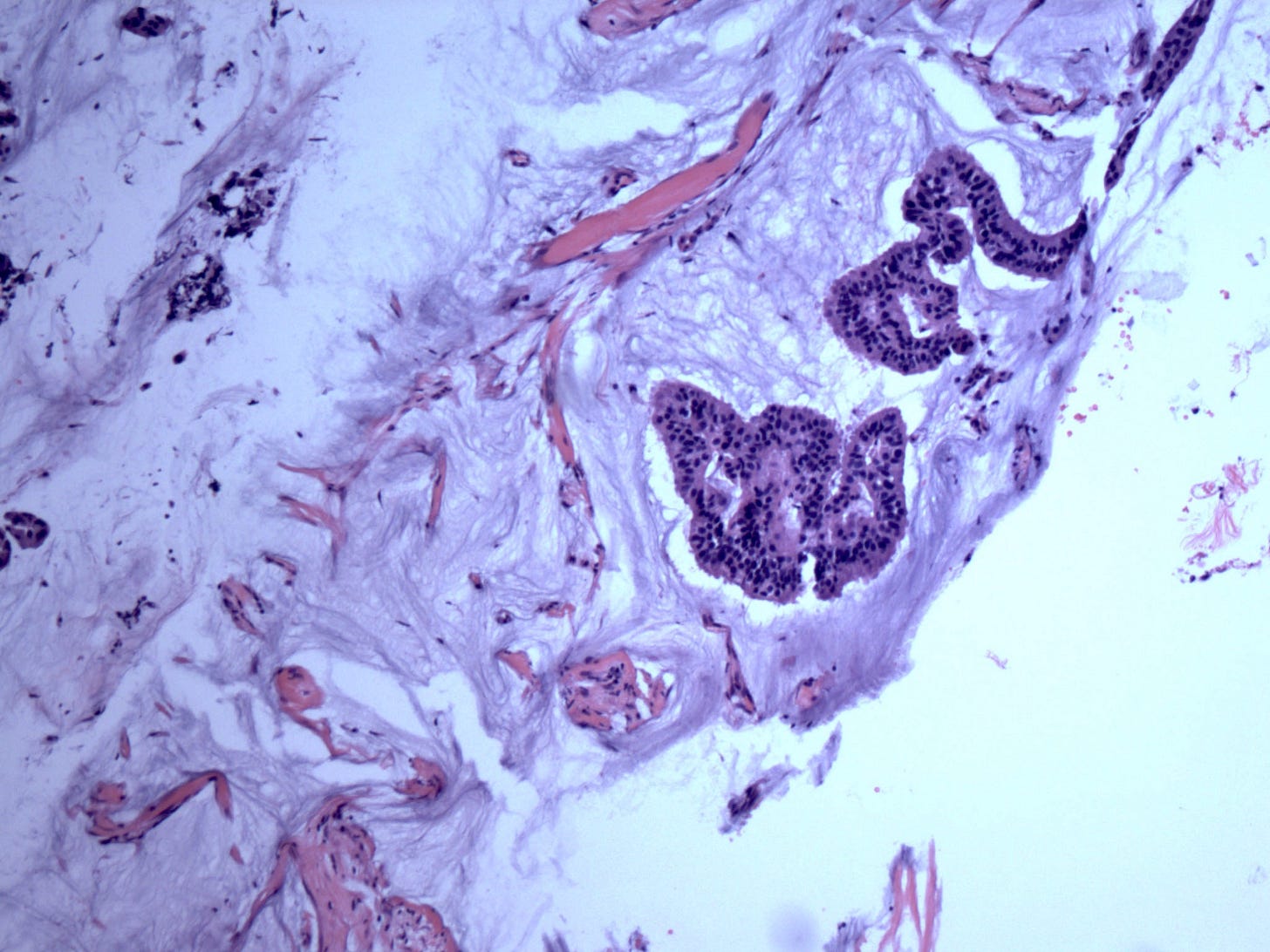
Mucinous ductal carcinoma in situ is a variant of DCIS, as previously discussed, with prominent intraluminal mucin:
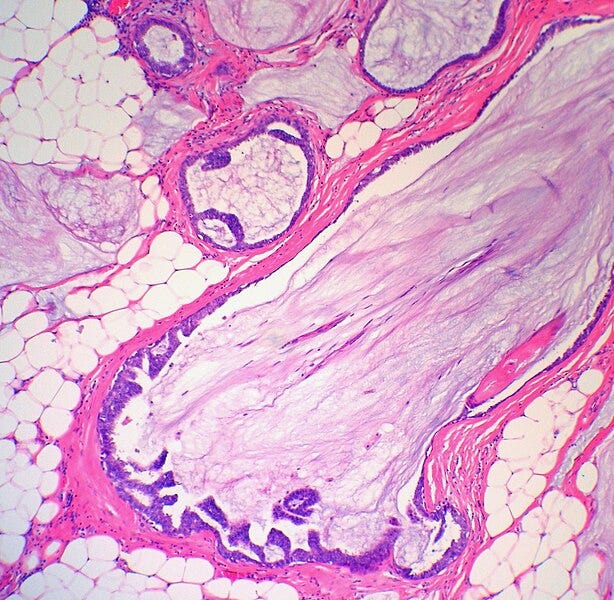
Mucocele-like lesion is a benign lesion composed of a ruptured cyst with detached strips of bland epithelium lined by myoepithelial cells floating within extravasated mucin:
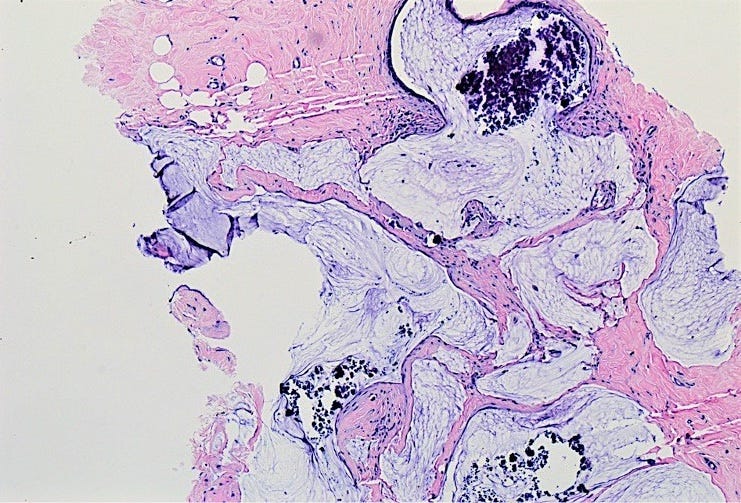
Solid papillary carcinoma, as previously discussed, commonly has intracellular and extracellular mucin and occasionally has signet ring cells:
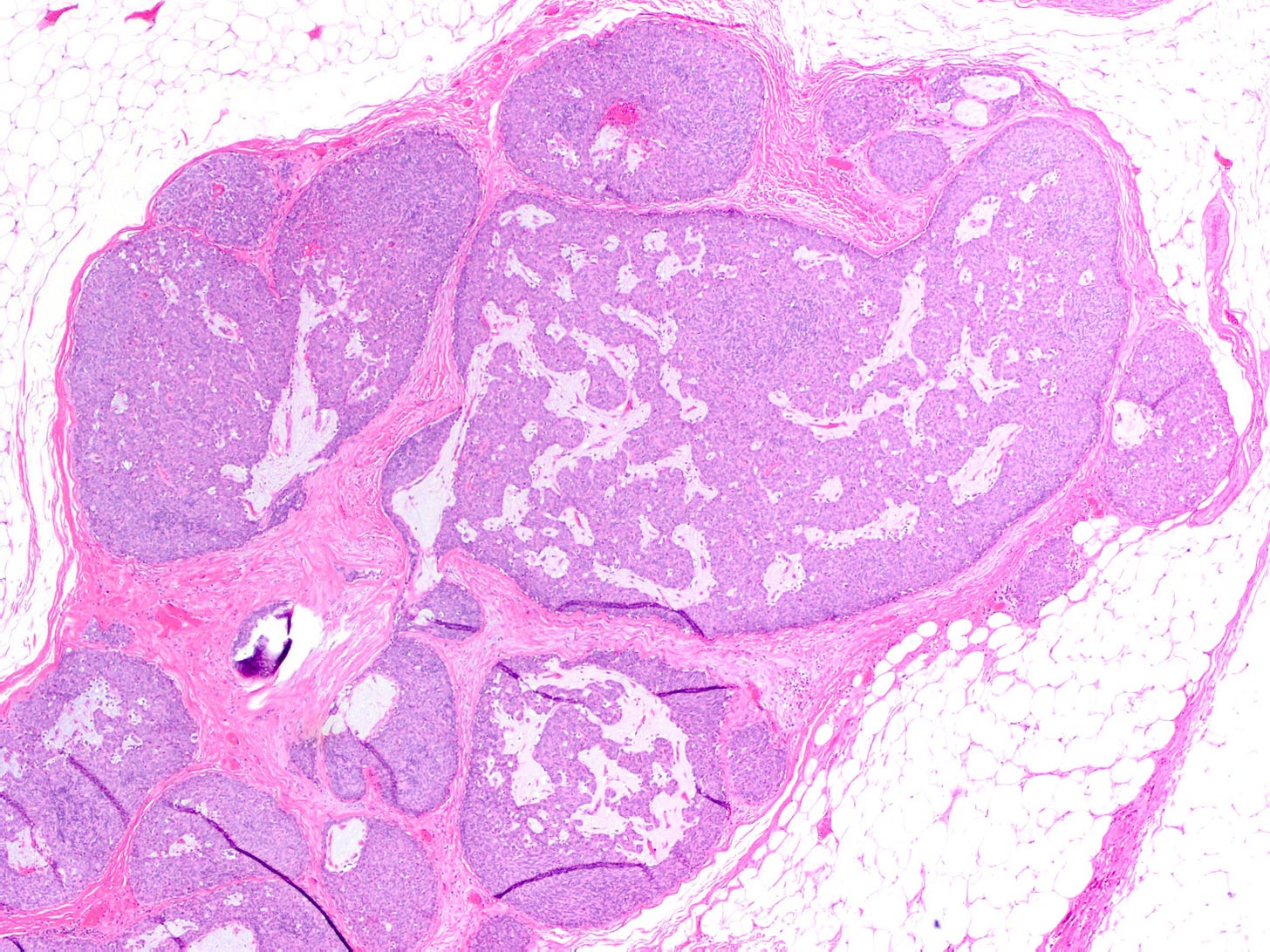
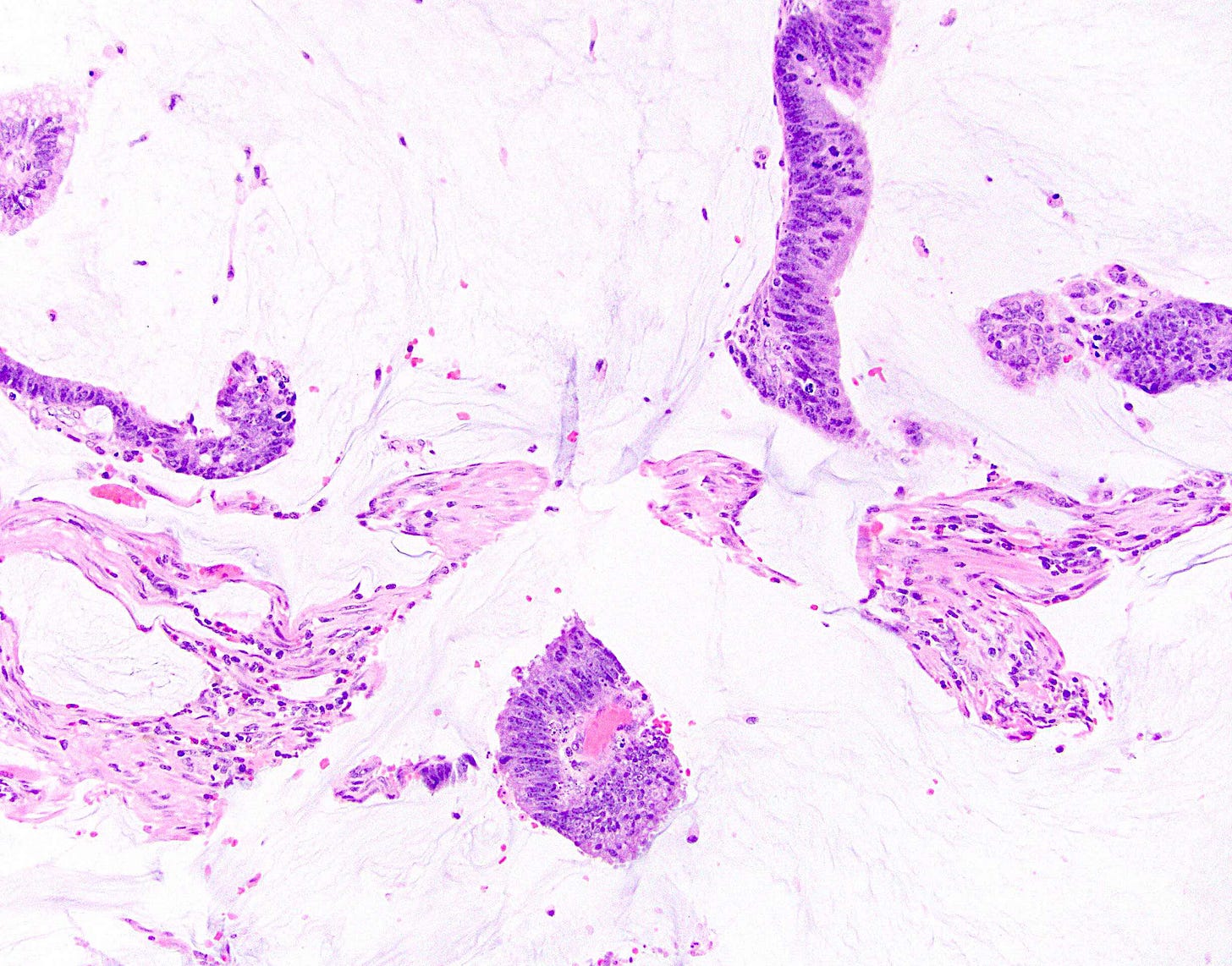
In addition, metastases to the breast from the gastrointestinal tract, lung or ovary may be mucinous:
The next essay will discuss other breast cancers without known precursors.
If you like these essays, please subscribe or share them with others.
Click here for the Index to Nat’s blog on Cancer and Medicine.
Follow me at https://www.linkedin.com/in/nat-pernick-8967765/ (LinkedIn), npernickmich (Threads and Instagram), natpernick.bsky.social (Bluesky) or @nat385440b (Tribel).
Follow our Curing Cancer Network through our Curing Cancer Newsletter, on LinkedIn or the CCN section of our PathologyOutlines.com blog. Each week we post interesting cancer related images of malignancies with diagnoses plus articles of interest. Please also read our CCN essays.
Latest versions of our cancer related documents:
American Code Against Cancer (how you can prevent cancer)
Email me at Nat@PathologyOutlines.com - Unfortunately, I cannot provide medical advice.
I also publish Notes at https://substack.com/note. Subscribers will automatically see my Notes.


